In this age of electronic devices, in which screens are the norm The appeal of tangible printed objects isn't diminished. It doesn't matter if it's for educational reasons as well as creative projects or just adding an individual touch to your space, Why Is It Called Dynamic Equilibrium can be an excellent resource. In this article, we'll dive into the world "Why Is It Called Dynamic Equilibrium," exploring the different types of printables, where to get them, as well as how they can improve various aspects of your life.
Get Latest Why Is It Called Dynamic Equilibrium Below

Why Is It Called Dynamic Equilibrium
Why Is It Called Dynamic Equilibrium - Why Is It Called Dynamic Equilibrium, Why Is Chemical Equilibrium Called Dynamic Equilibrium, Why Is Homeostasis Called A Dynamic Equilibrium, Why Is Dynamic Equilibrium Called Dynamic, Why Is Equilibrium Considered Dynamic, Why Is Equilibrium Dynamic
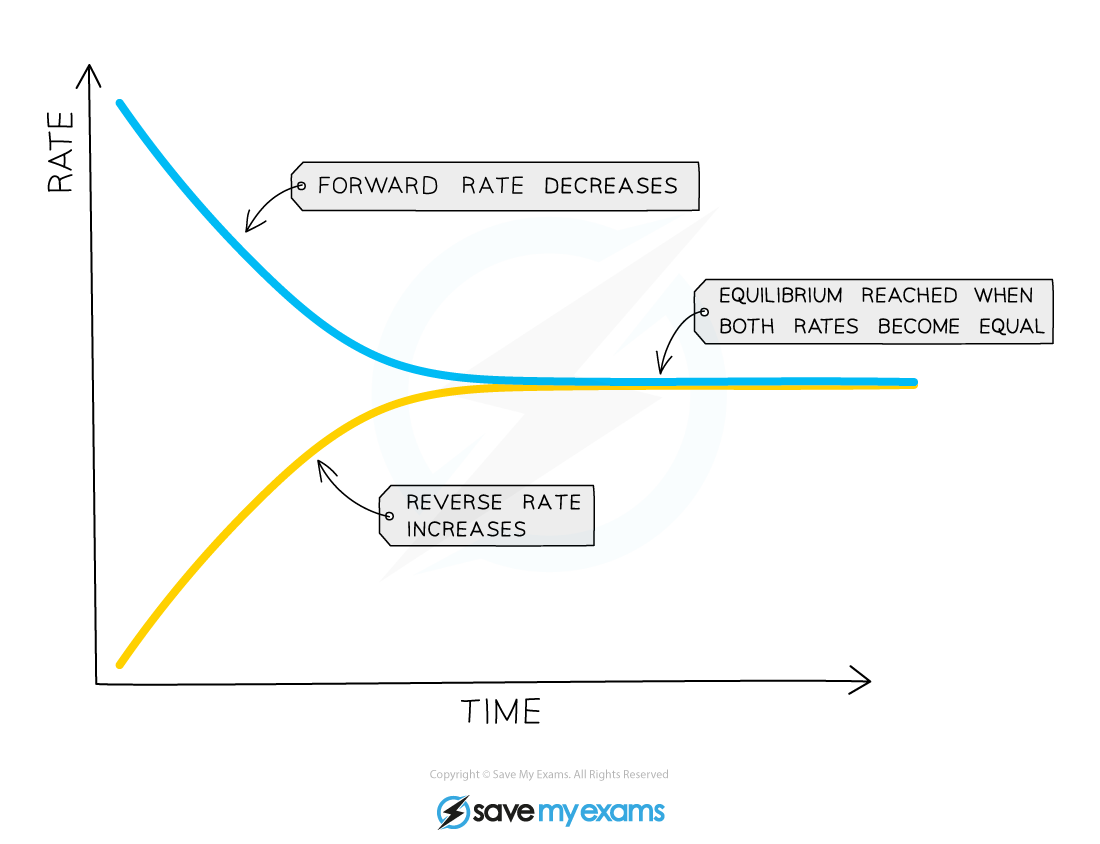
Dynamic equilibrium can be defined as the steady state of a reversible reaction occurring in a closed system Both the forward and reverse reaction proceeds in the opposite direction with equal rates
Therefore the dynamic equilibrium can be defined as A chemical reaction in which the rate of the reactants is equal to the rate of backward products In other words A reaction is said to be at dynamic equilibrium when the reactants are converted into products and the products are converted to reactants at an equal and constant rate
Why Is It Called Dynamic Equilibrium include a broad assortment of printable, downloadable content that can be downloaded from the internet at no cost. These printables come in different types, such as worksheets templates, coloring pages and many more. The appeal of printables for free is in their variety and accessibility.
More of Why Is It Called Dynamic Equilibrium
Edexcel IGCSE Chemistry 3 3 2 Dynamic Equilibrium
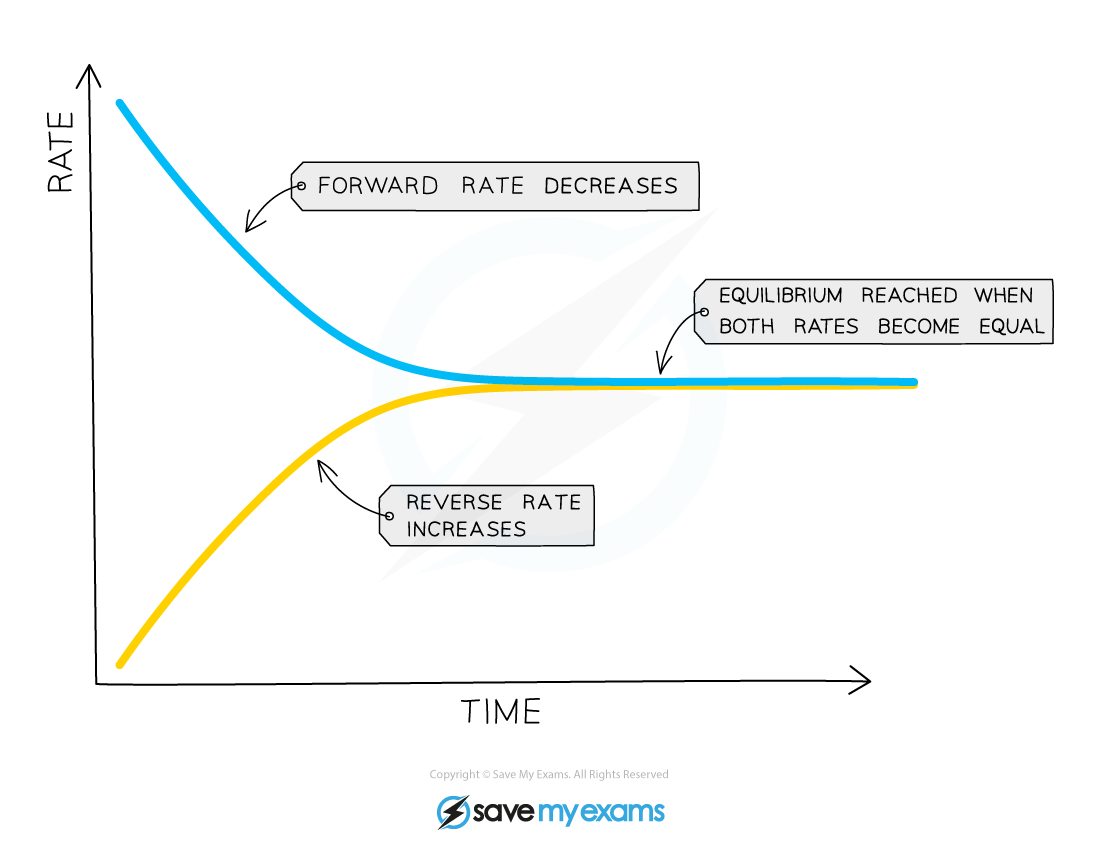
Edexcel IGCSE Chemistry 3 3 2 Dynamic Equilibrium
In chemistry a dynamic equilibrium exists once a reversible reaction occurs Substances transition between the reactants and products at equal rates meaning there is no net change Reactants and products are formed at such a rate that the concentration of neither changes It is a particular example of a system in a steady state
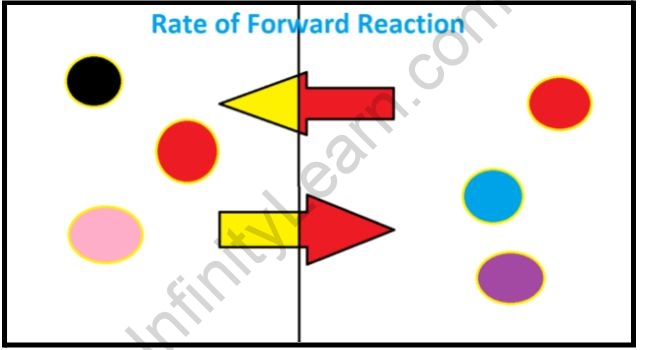
Chemical equilibrium is a dynamic process that consists of a forward reaction in which reactants are converted to products and a reverse reaction in which products are converted to reactants At equilibrium the forward and reverse reactions proceed at equal rates
The Why Is It Called Dynamic Equilibrium have gained huge popularity because of a number of compelling causes:
-
Cost-Efficiency: They eliminate the need to buy physical copies of the software or expensive hardware.
-
Individualization The Customization feature lets you tailor print-ready templates to your specific requirements when it comes to designing invitations as well as organizing your calendar, or decorating your home.
-
Educational Benefits: The free educational worksheets are designed to appeal to students of all ages. This makes them an essential tool for parents and teachers.
-
Simple: Quick access to a variety of designs and templates, which saves time as well as effort.
Where to Find more Why Is It Called Dynamic Equilibrium
Dynamic Equilibrium Definition Important Examples
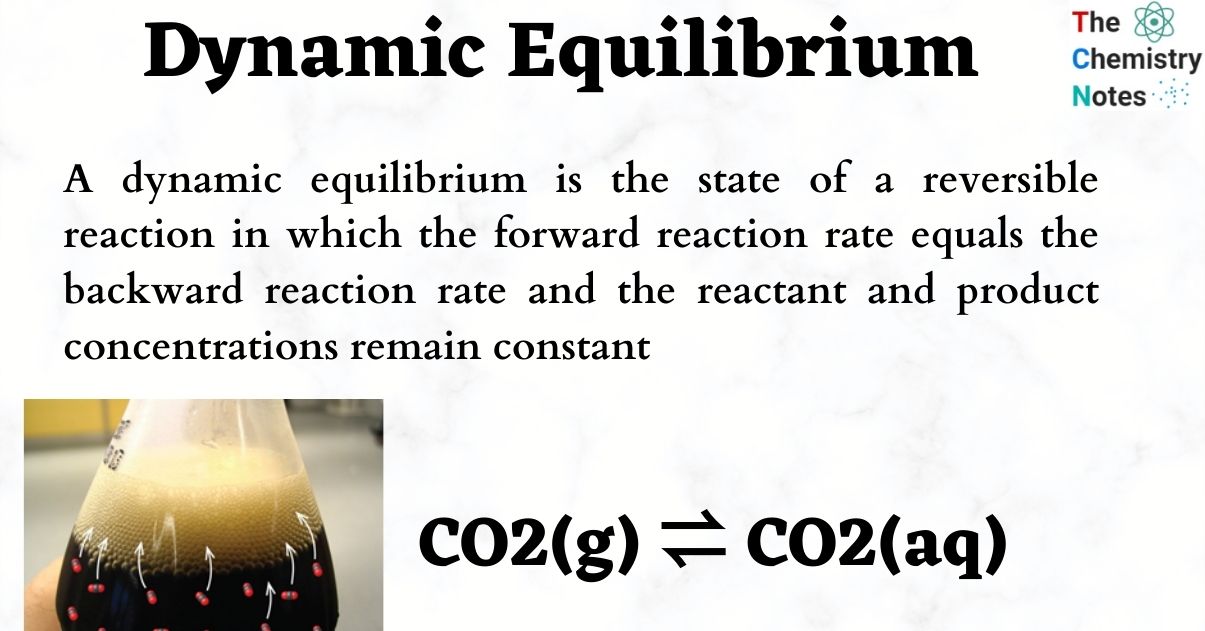
Dynamic Equilibrium Definition Important Examples
A dynamic equilibrium is established when the forward and reverse reactions of a reversible reaction occur at the same constant rate At dynamic equilibrium the concentration of the reactants and products remain constant even though both the forward and reverse reactions continue to occur
Many chemical reactions can easily run in both forward and reverse directions and are called reversible reactions The consequences of this reversible behavior has become known as a chemical reaction coming to equilibrium
We hope we've stimulated your interest in printables for free we'll explore the places you can find these treasures:
1. Online Repositories
- Websites like Pinterest, Canva, and Etsy offer an extensive collection of Why Is It Called Dynamic Equilibrium designed for a variety uses.
- Explore categories such as furniture, education, craft, and organization.
2. Educational Platforms
- Educational websites and forums usually offer worksheets with printables that are free as well as flashcards and other learning tools.
- Great for parents, teachers as well as students searching for supplementary sources.
3. Creative Blogs
- Many bloggers share their creative designs and templates at no cost.
- The blogs are a vast range of interests, everything from DIY projects to party planning.
Maximizing Why Is It Called Dynamic Equilibrium
Here are some fresh ways to make the most of printables for free:
1. Home Decor
- Print and frame beautiful images, quotes, or seasonal decorations to adorn your living areas.
2. Education
- Use free printable worksheets to enhance your learning at home and in class.
3. Event Planning
- Create invitations, banners, and other decorations for special occasions such as weddings, birthdays, and other special occasions.
4. Organization
- Stay organized with printable planners checklists for tasks, as well as meal planners.
Conclusion
Why Is It Called Dynamic Equilibrium are an abundance of practical and imaginative resources catering to different needs and hobbies. Their availability and versatility make these printables a useful addition to both personal and professional life. Explore the world of Why Is It Called Dynamic Equilibrium and open up new possibilities!
Frequently Asked Questions (FAQs)
-
Are printables actually for free?
- Yes they are! You can download and print these items for free.
-
Can I use free printables for commercial uses?
- It's determined by the specific terms of use. Make sure you read the guidelines for the creator prior to using the printables in commercial projects.
-
Do you have any copyright issues when you download Why Is It Called Dynamic Equilibrium?
- Certain printables might have limitations on their use. Always read the terms and regulations provided by the creator.
-
How can I print printables for free?
- You can print them at home with your printer or visit an area print shop for higher quality prints.
-
What software do I need to run Why Is It Called Dynamic Equilibrium?
- The majority of printables are in the format PDF. This is open with no cost programs like Adobe Reader.
Chemical Equilibrium Definition Principles And Examples

Why Is Dynamic Used To Describe Chemical Equilibrium

Check more sample of Why Is It Called Dynamic Equilibrium below
Dynamic Nature Of Equilibrium Infinity Learn By Sri Chaitanya
Chemical Equilibrium I Types Of Equilibrium Equilibrium Constant

Static And Dynamic Equilibrium Explained With Their Differences

Predicting Changes In Equilibrium Price And Quantity Outlier
Solved Experiments Have Shown That The Equilibrium Constant Kp For
Equilibrium Noticing The World The Reverse Gear


https://chemdictionary.org/dynamic-equilibrium
Therefore the dynamic equilibrium can be defined as A chemical reaction in which the rate of the reactants is equal to the rate of backward products In other words A reaction is said to be at dynamic equilibrium when the reactants are converted into products and the products are converted to reactants at an equal and constant rate
https://chem.libretexts.org/Bookshelves...
Chemical equilibrium occurs when the number of particles becoming products is equal to the number of particles becoming reactants A dynamic equilibrium is a state where the rate of the forward reaction is equal to the rate of the reverse reaction
Therefore the dynamic equilibrium can be defined as A chemical reaction in which the rate of the reactants is equal to the rate of backward products In other words A reaction is said to be at dynamic equilibrium when the reactants are converted into products and the products are converted to reactants at an equal and constant rate
Chemical equilibrium occurs when the number of particles becoming products is equal to the number of particles becoming reactants A dynamic equilibrium is a state where the rate of the forward reaction is equal to the rate of the reverse reaction

Predicting Changes In Equilibrium Price And Quantity Outlier

Chemical Equilibrium I Types Of Equilibrium Equilibrium Constant
Solved Experiments Have Shown That The Equilibrium Constant Kp For

Equilibrium Noticing The World The Reverse Gear

Difference Between Chemical Equilibrium And Dynamic Equilibrium

Physical Equilibrium Types Phase And Vapour liquid Equilibrium

Physical Equilibrium Types Phase And Vapour liquid Equilibrium

Difference Between Static And Dynamic Equilibrium Equilibrium