In the digital age, where screens rule our lives and our lives are dominated by screens, the appeal of tangible printed materials hasn't faded away. For educational purposes, creative projects, or simply to add an element of personalization to your space, Why Is Chemical Equilibrium Called Dynamic Equilibrium are now a vital source. Through this post, we'll take a dive in the world of "Why Is Chemical Equilibrium Called Dynamic Equilibrium," exploring what they are, where to get them, as well as how they can enhance various aspects of your lives.
Get Latest Why Is Chemical Equilibrium Called Dynamic Equilibrium Below
Why Is Chemical Equilibrium Called Dynamic Equilibrium
Why Is Chemical Equilibrium Called Dynamic Equilibrium -
Dynamic equilibrium In chemistry a dynamic equilibrium exists once a reversible reaction occurs Substances transition between the reactants and products at equal rates meaning there is no net change Reactants and products are formed at such a rate that the concentration of neither changes
Chemical equilibrium occurs when the number of particles becoming products is equal to the number of particles becoming reactants A dynamic equilibrium is a state where the rate of the forward reaction is equal to the rate of the reverse reaction
Printables for free include a vast range of printable, free content that can be downloaded from the internet at no cost. These resources come in various formats, such as worksheets, coloring pages, templates and many more. The value of Why Is Chemical Equilibrium Called Dynamic Equilibrium is in their variety and accessibility.
More of Why Is Chemical Equilibrium Called Dynamic Equilibrium
Chemical Equilibrium ClassNotes ng
Chemical Equilibrium ClassNotes ng
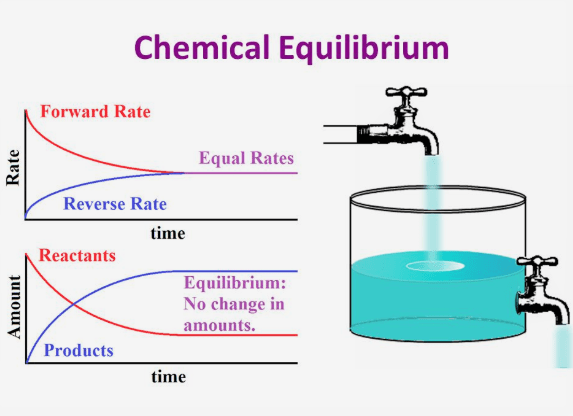
Revise equilibria including dynamic equilibrium the factors affecting equilibrium position temperature and pressure and catalysts
Many physical and chemical processes are reversible A reversible process is said to be in dynamic equilibrium when the forward and reverse processes occur at the same rate resulting in no observable change in the system
Why Is Chemical Equilibrium Called Dynamic Equilibrium have gained immense popularity due to numerous compelling reasons:
-
Cost-Effective: They eliminate the requirement of buying physical copies of the software or expensive hardware.
-
customization: Your HTML0 customization options allow you to customize the templates to meet your individual needs whether it's making invitations planning your schedule or decorating your home.
-
Educational Use: Educational printables that can be downloaded for free can be used by students of all ages, which makes them an essential instrument for parents and teachers.
-
An easy way to access HTML0: You have instant access a plethora of designs and templates is time-saving and saves effort.
Where to Find more Why Is Chemical Equilibrium Called Dynamic Equilibrium
Chemistry A Level Revision Equilibrium
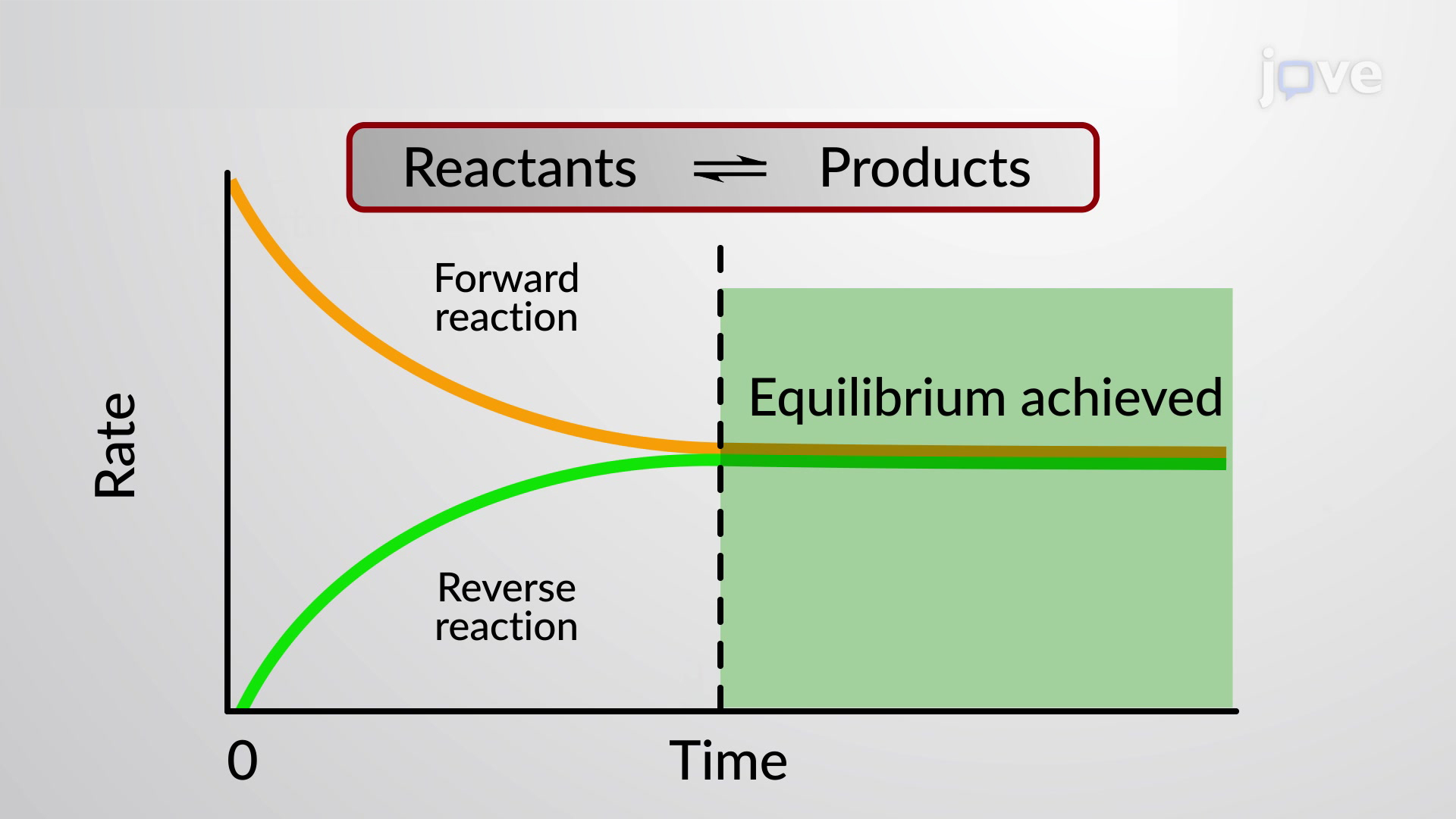
Chemistry A Level Revision Equilibrium
Chemical equilibrium is also called as dynamic equilibrium because it in chemical equilibrium the equilibrium can shift towards both sides that is reactant and product known as equilibrium shifting And in chemical equilibrium the reaction is going on but the rate of formation of reaction is equals to the rate of formation of product
A reaction is at equilibrium when the amounts of reactants or products no longer change Chemical equilibrium is a dynamic process meaning the rate of formation of products by the forward reaction is equal to the rate at which the products re form reactants by the reverse reaction
Now that we've ignited your interest in printables for free We'll take a look around to see where you can get these hidden treasures:
1. Online Repositories
- Websites like Pinterest, Canva, and Etsy offer a vast selection of Why Is Chemical Equilibrium Called Dynamic Equilibrium suitable for many uses.
- Explore categories like furniture, education, organizational, and arts and crafts.
2. Educational Platforms
- Educational websites and forums typically offer worksheets with printables that are free including flashcards, learning materials.
- Ideal for teachers, parents or students in search of additional resources.
3. Creative Blogs
- Many bloggers share their innovative designs and templates for free.
- The blogs covered cover a wide selection of subjects, that includes DIY projects to planning a party.
Maximizing Why Is Chemical Equilibrium Called Dynamic Equilibrium
Here are some ideas create the maximum value of printables for free:
1. Home Decor
- Print and frame gorgeous art, quotes, or seasonal decorations that will adorn your living areas.
2. Education
- Use printable worksheets from the internet to build your knowledge at home for the classroom.
3. Event Planning
- Invitations, banners and decorations for special occasions such as weddings, birthdays, and other special occasions.
4. Organization
- Be organized by using printable calendars as well as to-do lists and meal planners.
Conclusion
Why Is Chemical Equilibrium Called Dynamic Equilibrium are an abundance of creative and practical resources which cater to a wide range of needs and preferences. Their availability and versatility make them an essential part of each day life. Explore the vast world of Why Is Chemical Equilibrium Called Dynamic Equilibrium today to discover new possibilities!
Frequently Asked Questions (FAQs)
-
Do printables with no cost really free?
- Yes, they are! You can download and print these free resources for no cost.
-
Do I have the right to use free printables for commercial uses?
- It's based on specific rules of usage. Always review the terms of use for the creator before using any printables on commercial projects.
-
Do you have any copyright violations with printables that are free?
- Some printables may come with restrictions in use. Always read the terms and conditions set forth by the designer.
-
How can I print printables for free?
- Print them at home using a printer or visit a print shop in your area for high-quality prints.
-
What program will I need to access printables free of charge?
- Most PDF-based printables are available in PDF format. These is open with no cost software, such as Adobe Reader.
Chemical Equilibrum Passnownow
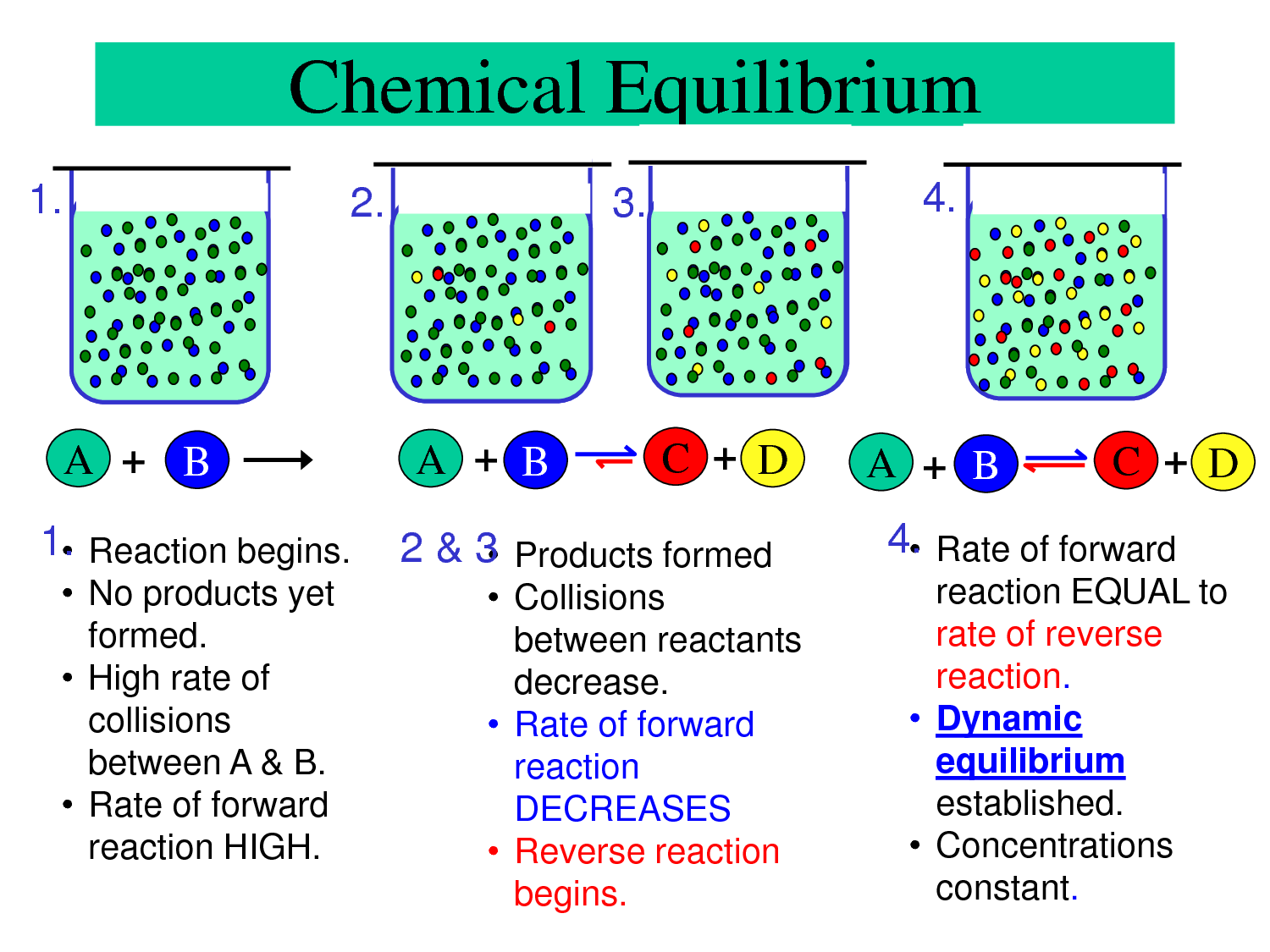
Chemical Equilibrium What Are The Characteristics Of Equilibrium
Check more sample of Why Is Chemical Equilibrium Called Dynamic Equilibrium below
Chemical Equilibrium 826 Plays Quizizz
Chemical Equilibrium

Chemical Equilibrium
Difference Between Chemical Equilibrium And Dynamic Equilibrium

Difference Between Static And Dynamic Equilibrium Equilibrium

Chemical Equilibrium Mindmeister Mind Map Gambaran
https:// chem.libretexts.org /Bookshelves...
Chemical equilibrium occurs when the number of particles becoming products is equal to the number of particles becoming reactants A dynamic equilibrium is a state where the rate of the forward reaction is equal to the rate of the reverse reaction

https:// socratic.org /questions/why-is-chemical-equilibrium-dynamic
Chemical equilibrium refers to the balance between products and reactants after a given reaction has reached a state of order in which both reactants and products are forming at a constant rate It is dynamic because there are many factors that affect what that ratio will be as defined by LeChatelier Heat
Chemical equilibrium occurs when the number of particles becoming products is equal to the number of particles becoming reactants A dynamic equilibrium is a state where the rate of the forward reaction is equal to the rate of the reverse reaction
Chemical equilibrium refers to the balance between products and reactants after a given reaction has reached a state of order in which both reactants and products are forming at a constant rate It is dynamic because there are many factors that affect what that ratio will be as defined by LeChatelier Heat

Difference Between Chemical Equilibrium And Dynamic Equilibrium

Chemical Equilibrium

Difference Between Static And Dynamic Equilibrium Equilibrium

Chemical Equilibrium Mindmeister Mind Map Gambaran

Equilibrium Made Easy How To Solve Chemical Equilibrium Problems YouTube

Dynamic Chemical Equilibrium Definition Examples Video Lesson

Dynamic Chemical Equilibrium Definition Examples Video Lesson
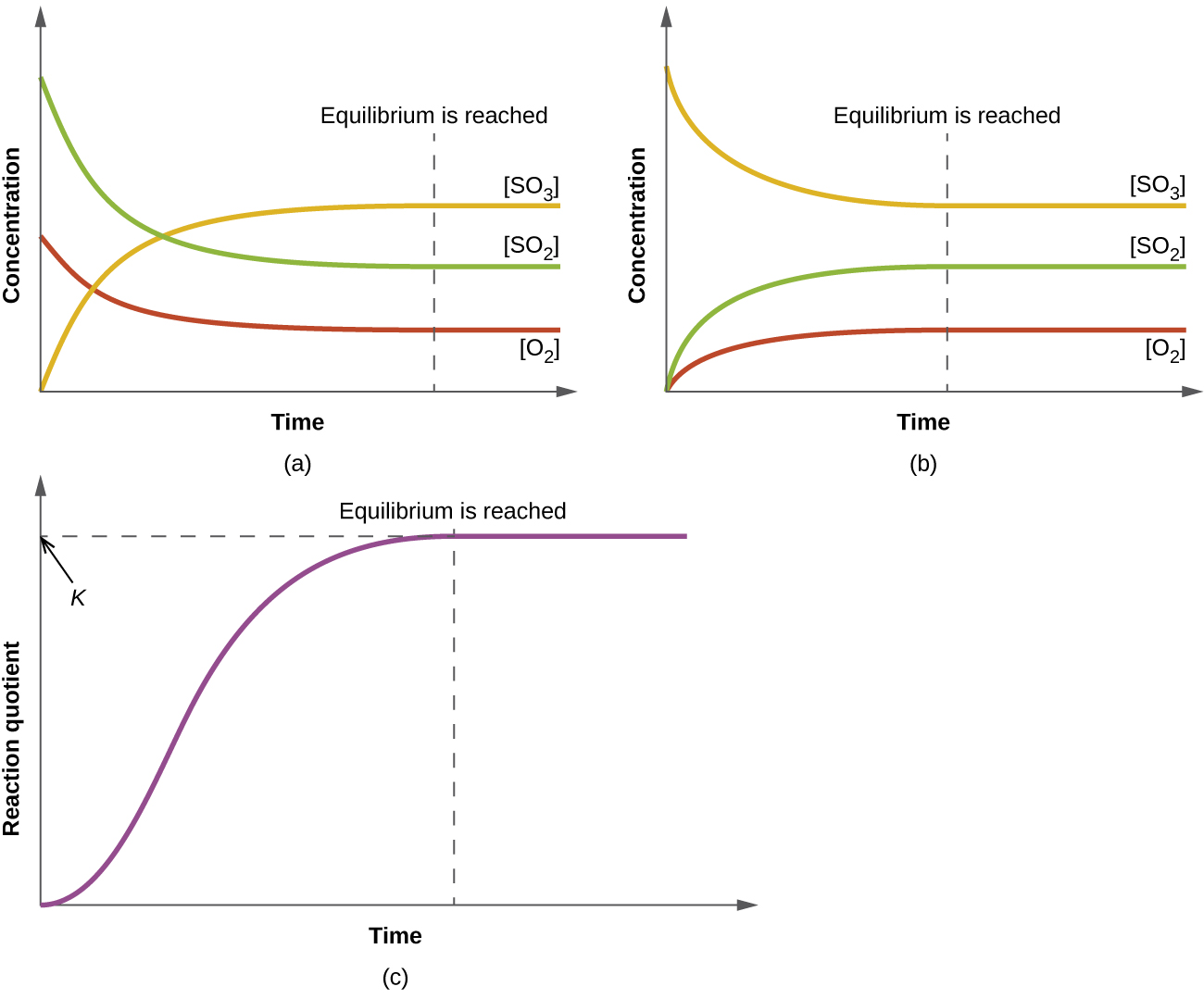
13 2 Equilibrium Constants Chemistry 112 Chapters 12 17 Of OpenStax