In the age of digital, when screens dominate our lives yet the appeal of tangible printed objects isn't diminished. Whether it's for educational purposes, creative projects, or simply adding an element of personalization to your area, Why Is Equilibrium Dynamic can be an excellent resource. With this guide, you'll take a dive into the sphere of "Why Is Equilibrium Dynamic," exploring the benefits of them, where they are, and ways they can help you improve many aspects of your daily life.
Get Latest Why Is Equilibrium Dynamic Below

Why Is Equilibrium Dynamic
Why Is Equilibrium Dynamic - Why Is Equilibrium Dynamic, Why Is Equilibrium Dynamic In Nature, Why Is Dynamic Equilibrium Important, Why Is Equilibrium Considered Dynamic, Why Is Equilibrium A Dynamic Process, Why Is Dynamic Balance Important, Why Chemical Equilibrium Is Dynamic Class 10, Why Is Equilibrium Called A Dynamic State, Why Chemical Equilibrium Is Dynamic In Nature Explain With Example, Equilibrium In Chemical Processes Dynamic Equilibrium
A reaction is at equilibrium when the amounts of reactants or products no longer change Chemical equilibrium is a dynamic process meaning the rate of formation of products by the forward reaction is equal to
Chemical equilibrium occurs when the number of particles becoming products is equal to the number of particles becoming reactants A dynamic equilibrium is a state where the rate of the forward reaction is equal
Printables for free cover a broad selection of printable and downloadable material that is available online at no cost. These resources come in various types, like worksheets, templates, coloring pages, and much more. The appeal of printables for free lies in their versatility and accessibility.
More of Why Is Equilibrium Dynamic
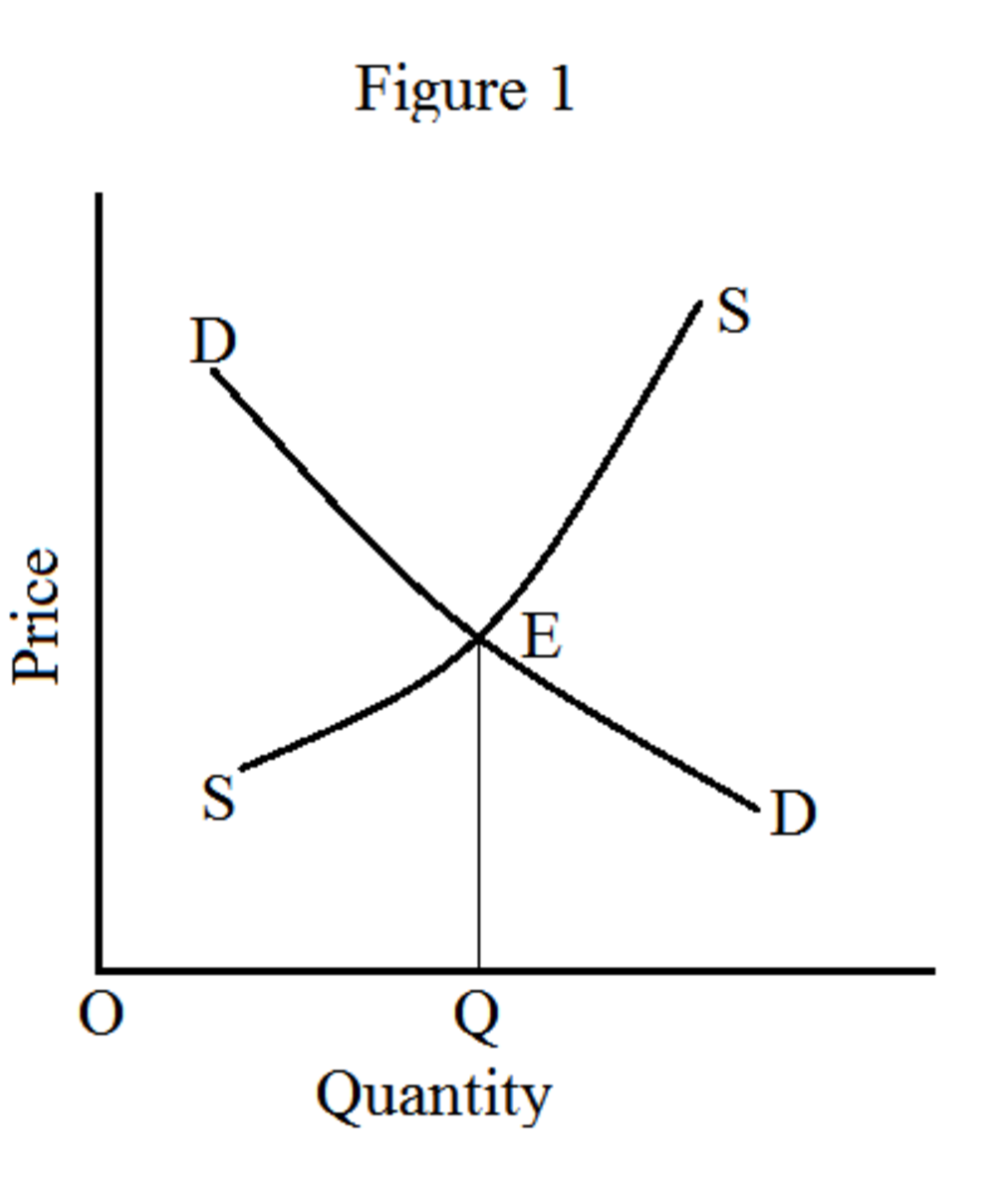
Equilibrium Price Definition Types Example And How To Calculate
:max_bytes(150000):strip_icc()/Equilibrium_Version1_4197021-488ad218b7a9456e96ea400c6588359c.png)
Equilibrium Price Definition Types Example And How To Calculate
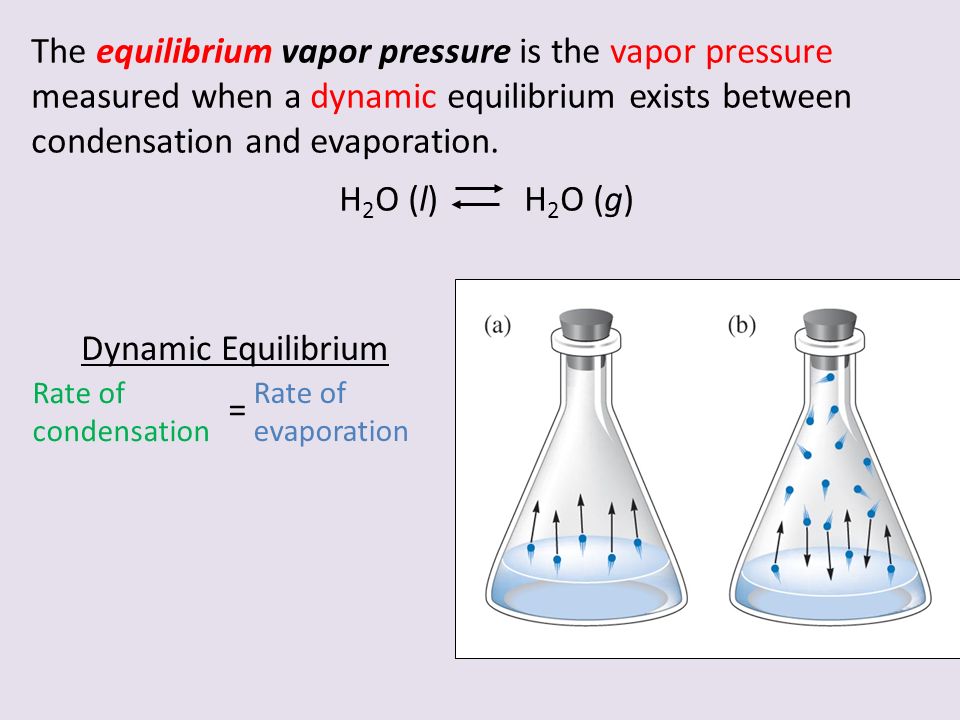
Dynamic equilibrium can be defined as the steady state of a reversible reaction occurring in a closed system Both the forward and reverse reaction proceeds in the opposite direction with equal rates
Chemical equilibrium is a dynamic process that consists of a forward reaction in which reactants are converted to products and a reverse reaction in which products are converted to reactants At equilibrium the
Why Is Equilibrium Dynamic have gained immense popularity for several compelling reasons:
-
Cost-Effective: They eliminate the need to purchase physical copies or expensive software.
-
Flexible: You can tailor printables to your specific needs in designing invitations and schedules, or decorating your home.
-
Educational Worth: Printables for education that are free offer a wide range of educational content for learners of all ages, making them a useful tool for parents and educators.
-
An easy way to access HTML0: The instant accessibility to an array of designs and templates saves time and effort.
Where to Find more Why Is Equilibrium Dynamic
13 1 Chemical Equilibria Chemistry 112 Chapters 12 17 Of OpenStax
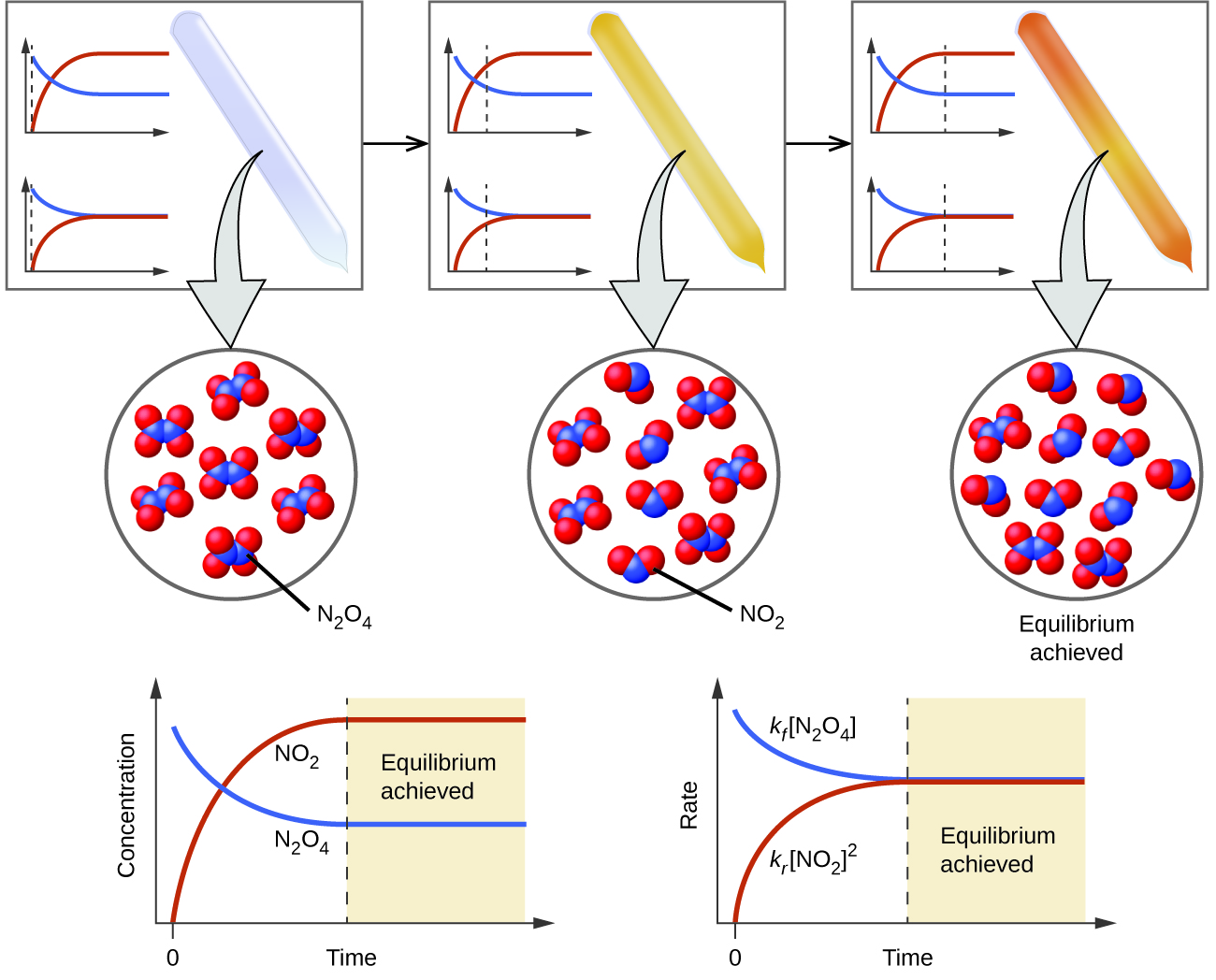
13 1 Chemical Equilibria Chemistry 112 Chapters 12 17 Of OpenStax
Chemical equilibrium and dynamic equilibrium are both concepts used to describe the state of a chemical reaction Chemical equilibrium refers to a state where the forward and reverse
The exact moment of equilibrium happens when the rate of the forward reaction equals the rate of the reverse reaction When a chemical system is at equilibrium there are no visible
Now that we've ignited your interest in Why Is Equilibrium Dynamic Let's look into where they are hidden treasures:
1. Online Repositories
- Websites such as Pinterest, Canva, and Etsy provide a wide selection in Why Is Equilibrium Dynamic for different needs.
- Explore categories such as decoration for your home, education, management, and craft.
2. Educational Platforms
- Forums and websites for education often provide worksheets that can be printed for free for flashcards, lessons, and worksheets. materials.
- Ideal for parents, teachers as well as students searching for supplementary resources.
3. Creative Blogs
- Many bloggers share their creative designs or templates for download.
- The blogs are a vast array of topics, ranging starting from DIY projects to party planning.
Maximizing Why Is Equilibrium Dynamic
Here are some unique ways how you could make the most of Why Is Equilibrium Dynamic:
1. Home Decor
- Print and frame stunning images, quotes, or seasonal decorations to adorn your living areas.
2. Education
- Use these printable worksheets free of charge to enhance your learning at home also in the classes.
3. Event Planning
- Invitations, banners and decorations for special occasions like weddings and birthdays.
4. Organization
- Stay organized with printable planners as well as to-do lists and meal planners.
Conclusion
Why Is Equilibrium Dynamic are an abundance filled with creative and practical information that cater to various needs and preferences. Their availability and versatility make them a fantastic addition to each day life. Explore the world of Why Is Equilibrium Dynamic today to open up new possibilities!
Frequently Asked Questions (FAQs)
-
Are Why Is Equilibrium Dynamic really completely free?
- Yes you can! You can print and download these documents for free.
-
Does it allow me to use free printouts for commercial usage?
- It is contingent on the specific terms of use. Always consult the author's guidelines before utilizing printables for commercial projects.
-
Are there any copyright problems with printables that are free?
- Some printables could have limitations in use. Be sure to check the terms and conditions offered by the author.
-
How can I print Why Is Equilibrium Dynamic?
- You can print them at home with either a printer or go to a local print shop to purchase premium prints.
-
What software do I require to open printables at no cost?
- Most PDF-based printables are available in PDF format, which can be opened using free programs like Adobe Reader.
Why Is Dynamic Used To Describe Chemical Equilibrium

W02M04 Dynamic Equilibrium Equation By Energy Method YouTube

Check more sample of Why Is Equilibrium Dynamic below
Chemical Equilibrium 826 Plays Quizizz
What Do We Mean By A Dynamic Equilibrium Can You Describe How The
Static And Dynamic Equilibrium HubPages
Physiology Of Equilibrium Balance
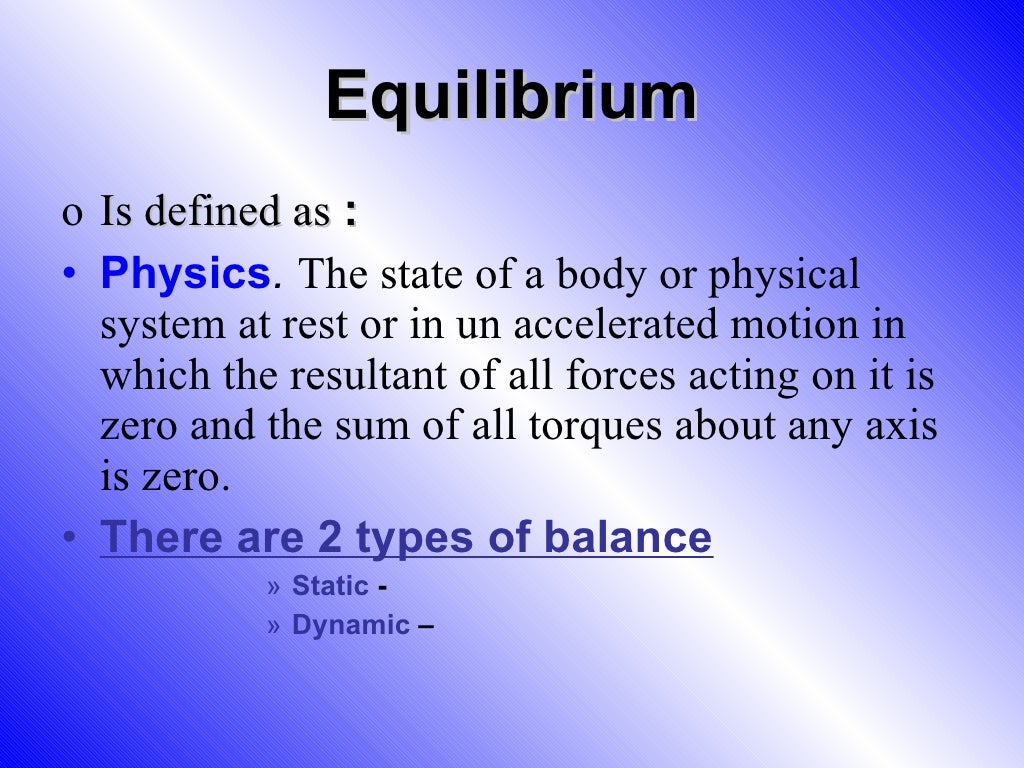
Difference Between Static And Dynamic Equilibrium Equilibrium

Static And Dynamic Equilibrium Explained With Their Differences


https://chem.libretexts.org/Bookshelves...
Chemical equilibrium occurs when the number of particles becoming products is equal to the number of particles becoming reactants A dynamic equilibrium is a state where the rate of the forward reaction is equal
:max_bytes(150000):strip_icc()/Equilibrium_Version1_4197021-488ad218b7a9456e96ea400c6588359c.png?w=186)
https://chemdictionary.org/dynamic-equ…
A reaction is said to be at dynamic equilibrium when the reactants are converted into products and the products are converted to reactants at an equal and constant rate So the equilibrium is the state of equal and
Chemical equilibrium occurs when the number of particles becoming products is equal to the number of particles becoming reactants A dynamic equilibrium is a state where the rate of the forward reaction is equal
A reaction is said to be at dynamic equilibrium when the reactants are converted into products and the products are converted to reactants at an equal and constant rate So the equilibrium is the state of equal and

Physiology Of Equilibrium Balance

What Do We Mean By A Dynamic Equilibrium Can You Describe How The

Difference Between Static And Dynamic Equilibrium Equilibrium

Static And Dynamic Equilibrium Explained With Their Differences

PPT Equilibrium

Equilibrium Equilibrium Photo 22461181 Fanpop

Equilibrium Equilibrium Photo 22461181 Fanpop

Chemical Equilibrium Dynamic Equilibrium In Chemistry