In a world where screens dominate our lives and the appeal of physical printed objects hasn't waned. Whatever the reason, whether for education or creative projects, or simply to add an individual touch to the home, printables for free have become an invaluable source. In this article, we'll dive in the world of "Why Is Equilibrium A Dynamic Process," exploring what they are, where to find them and how they can enhance various aspects of your life.
Get Latest Why Is Equilibrium A Dynamic Process Below
:max_bytes(150000):strip_icc()/Equilibrium_Version1_4197021-488ad218b7a9456e96ea400c6588359c.png)
Why Is Equilibrium A Dynamic Process
Why Is Equilibrium A Dynamic Process - Why Is Equilibrium A Dynamic Process, Why Is Equilibrium Considered Dynamic Process, Why Is Chemical Equilibrium Described As A Dynamic Process, Equilibrium In Chemical Processes Dynamic Equilibrium, Equilibrium Is A Dynamic Process Because The, Why Is Equilibrium Dynamic, Why Is Equilibrium Considered Dynamic, What Happens During Dynamic Equilibrium
Explanation of dynamic equilibrium Consider an object is moving at a constant speed that balances all the forces on that object It will show a dynamic equilibrium when a horizontal force is applied to an object and cause
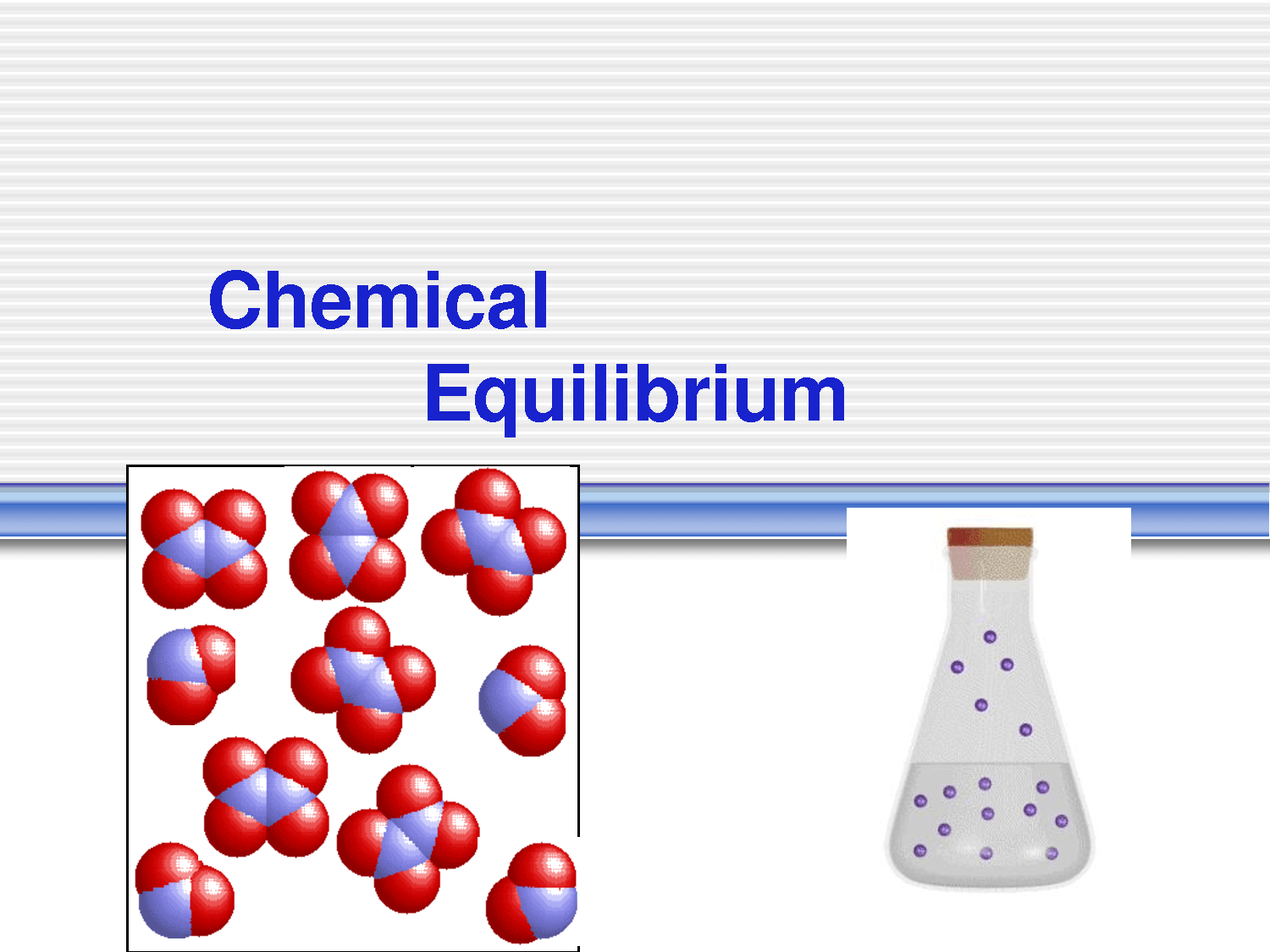
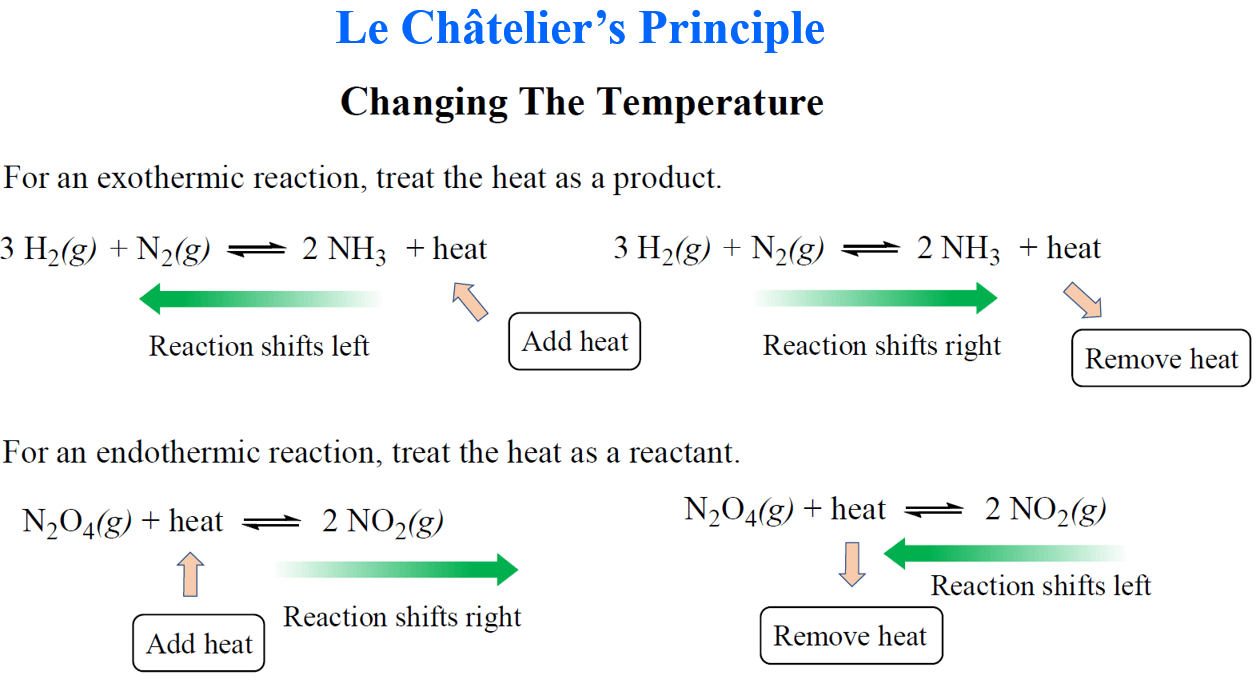
Chemical equilibrium refers to the balance between products and reactants after a given reaction has reached a state of order in which both reactants and products are forming
Printables for free cover a broad assortment of printable resources available online for download at no cost. These resources come in many formats, such as worksheets, templates, coloring pages and more. One of the advantages of Why Is Equilibrium A Dynamic Process lies in their versatility as well as accessibility.
More of Why Is Equilibrium A Dynamic Process
Chemistry A Level Revision Equilibrium
Chemistry A Level Revision Equilibrium
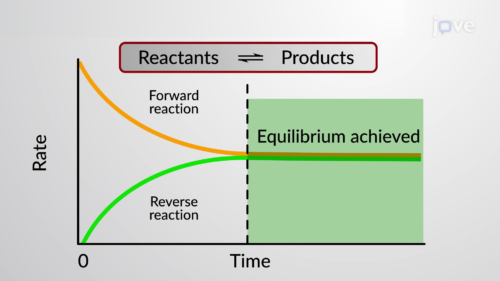
Dynamic equilibrium can be defined as the steady state of a reversible reaction occurring in a closed system Both the forward and reverse reaction proceeds in the opposite direction with equal rates
Dynamic Equilibrium can be defined as the state of a given system in which the reversible reaction taking place in it stops changing the ratio of reactants and products but there is a movement of substances between the reactants and
Print-friendly freebies have gained tremendous recognition for a variety of compelling motives:
-
Cost-Efficiency: They eliminate the necessity to purchase physical copies of the software or expensive hardware.
-
customization We can customize print-ready templates to your specific requirements whether you're designing invitations, organizing your schedule, or even decorating your house.
-
Educational Benefits: Printing educational materials for no cost provide for students of all ages. This makes them an invaluable tool for parents and educators.
-
Convenience: Quick access to various designs and templates reduces time and effort.
Where to Find more Why Is Equilibrium A Dynamic Process
Why Is Dynamic Used To Describe Chemical Equilibrium

Why Is Dynamic Used To Describe Chemical Equilibrium
Chemical equilibrium occurs when the number of particles becoming products is equal to the number of particles becoming reactants A dynamic equilibrium is a state where the rate of the forward reaction is equal
Dynamic equilibrium in chemistry means that reactants are constantly forming products and products are constantly forming reactants Since the rates of formation are identical
We've now piqued your curiosity about Why Is Equilibrium A Dynamic Process Let's see where you can get these hidden treasures:
1. Online Repositories
- Websites like Pinterest, Canva, and Etsy offer a vast selection and Why Is Equilibrium A Dynamic Process for a variety motives.
- Explore categories such as decoration for your home, education, management, and craft.
2. Educational Platforms
- Forums and websites for education often provide free printable worksheets, flashcards, and learning materials.
- Great for parents, teachers and students looking for extra resources.
3. Creative Blogs
- Many bloggers are willing to share their original designs and templates for no cost.
- The blogs covered cover a wide spectrum of interests, everything from DIY projects to party planning.
Maximizing Why Is Equilibrium A Dynamic Process
Here are some ways in order to maximize the use use of Why Is Equilibrium A Dynamic Process:
1. Home Decor
- Print and frame beautiful artwork, quotes, or seasonal decorations to adorn your living spaces.
2. Education
- Use printable worksheets from the internet to build your knowledge at home (or in the learning environment).
3. Event Planning
- Design invitations for banners, invitations and decorations for special events like weddings or birthdays.
4. Organization
- Keep your calendars organized by printing printable calendars including to-do checklists, daily lists, and meal planners.
Conclusion
Why Is Equilibrium A Dynamic Process are a treasure trove of creative and practical resources that cater to various needs and interests. Their accessibility and versatility make they a beneficial addition to the professional and personal lives of both. Explore the many options of Why Is Equilibrium A Dynamic Process and uncover new possibilities!
Frequently Asked Questions (FAQs)
-
Are Why Is Equilibrium A Dynamic Process truly absolutely free?
- Yes they are! You can print and download these materials for free.
-
Do I have the right to use free templates for commercial use?
- It depends on the specific conditions of use. Be sure to read the rules of the creator prior to printing printables for commercial projects.
-
Do you have any copyright rights issues with Why Is Equilibrium A Dynamic Process?
- Some printables may come with restrictions in use. Make sure to read the terms and conditions provided by the creator.
-
How can I print Why Is Equilibrium A Dynamic Process?
- You can print them at home using either a printer at home or in the local print shops for the highest quality prints.
-
What software will I need to access Why Is Equilibrium A Dynamic Process?
- Most printables come in the format PDF. This can be opened using free programs like Adobe Reader.
Le Chateliers Principle Steps Of Chemistry 2022
Chemical Equilibrium 826 Plays Quizizz
Check more sample of Why Is Equilibrium A Dynamic Process below
Chemical Equilibrium What Are The Characteristics Of Equilibrium
Explain Equilibrium Price How Is It Determined
Static And Dynamic Equilibrium Explained With Their Differences

What Is Equilibrium Diagram Photos

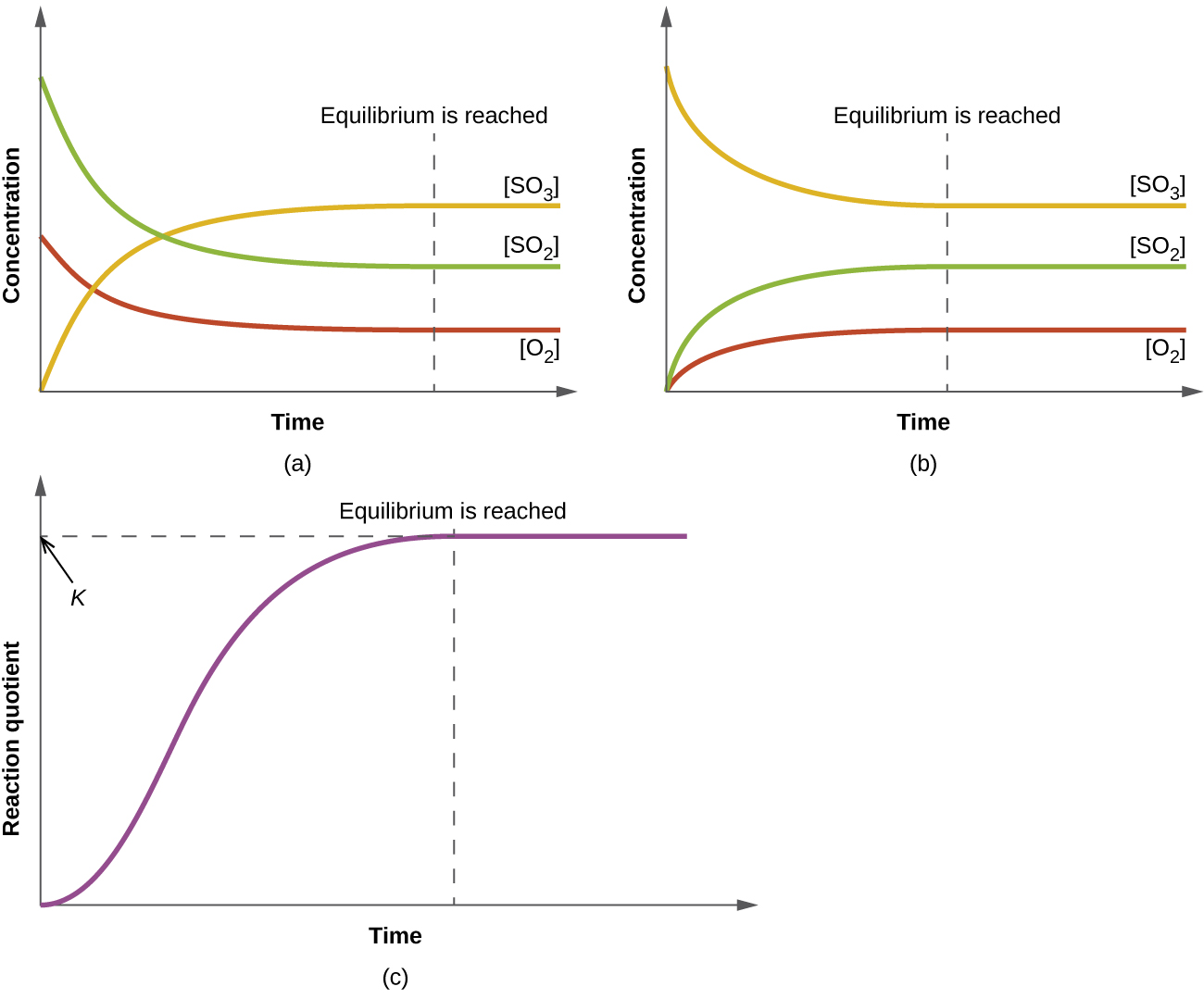
13 2 Equilibrium Constants Chemistry 112 Chapters 12 17 Of OpenStax
W02M04 Dynamic Equilibrium Equation By Energy Method YouTube

:max_bytes(150000):strip_icc()/Equilibrium_Version1_4197021-488ad218b7a9456e96ea400c6588359c.png?w=186)
https://socratic.org/questions/why-is-chemical-equilibrium-dynamic
Chemical equilibrium refers to the balance between products and reactants after a given reaction has reached a state of order in which both reactants and products are forming

https://chem.libretexts.org/Courses/Oreg…
A reaction is at equilibrium when the amounts of reactants or products no longer change Chemical equilibrium is a dynamic process meaning the rate of formation of products by the forward reaction is equal to
Chemical equilibrium refers to the balance between products and reactants after a given reaction has reached a state of order in which both reactants and products are forming
A reaction is at equilibrium when the amounts of reactants or products no longer change Chemical equilibrium is a dynamic process meaning the rate of formation of products by the forward reaction is equal to

What Is Equilibrium Diagram Photos

Explain Equilibrium Price How Is It Determined

13 2 Equilibrium Constants Chemistry 112 Chapters 12 17 Of OpenStax

W02M04 Dynamic Equilibrium Equation By Energy Method YouTube

Chemical Equilibrium Dynamic Equilibrium In Chemistry

Object In Equilibrium

Object In Equilibrium

Static Equilibrium Worksheet With Answers Pdf Must See Magna Worksheets