In the digital age, where screens rule our lives and the appeal of physical printed materials isn't diminishing. No matter whether it's for educational uses and creative work, or just adding an individual touch to your space, Why Is Chemical Equilibrium Described As A Dynamic Process are now an essential resource. The following article is a dive deep into the realm of "Why Is Chemical Equilibrium Described As A Dynamic Process," exploring the different types of printables, where to find them and the ways that they can benefit different aspects of your life.
Get Latest Why Is Chemical Equilibrium Described As A Dynamic Process Below
Why Is Chemical Equilibrium Described As A Dynamic Process
Why Is Chemical Equilibrium Described As A Dynamic Process -
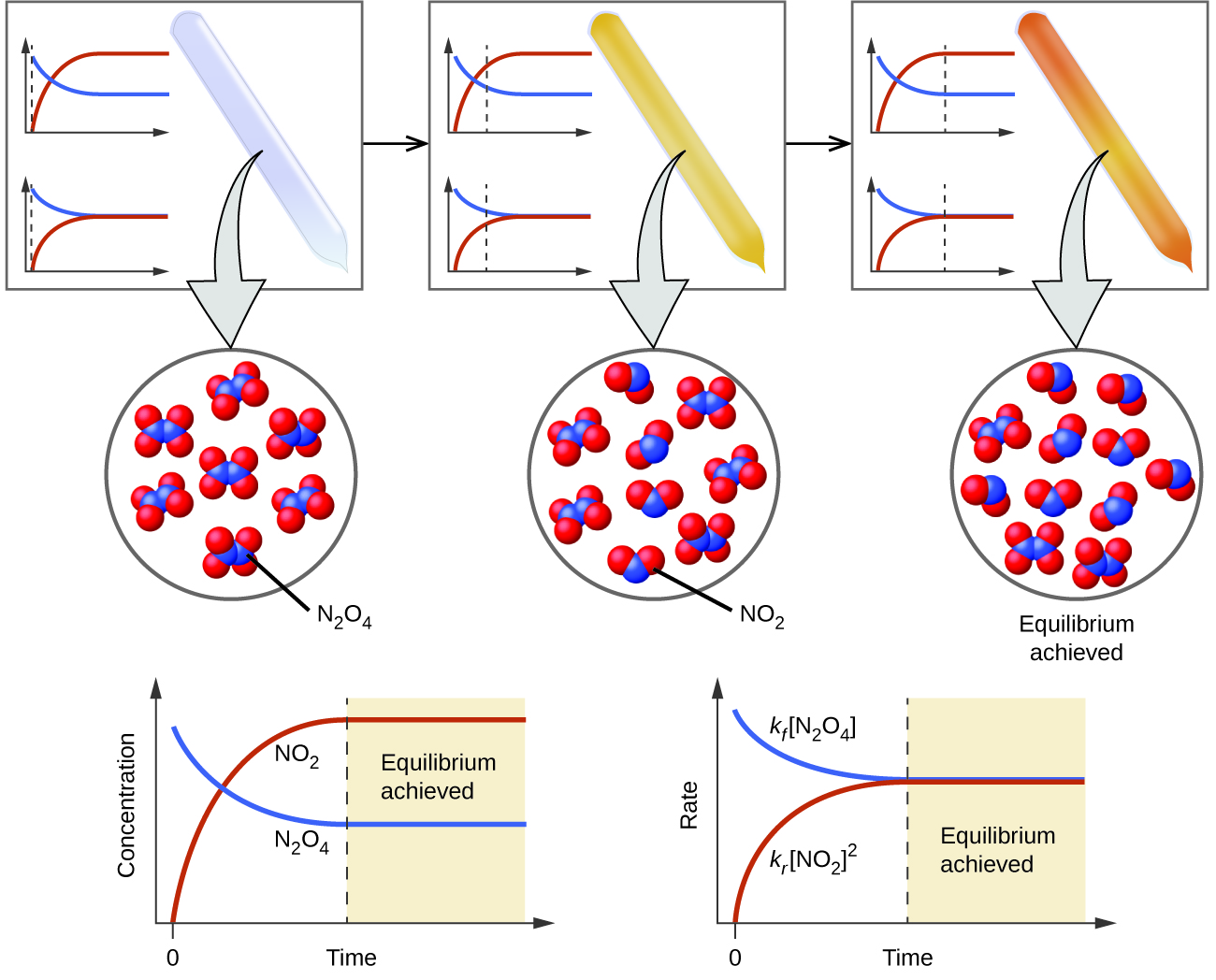
At such a stage the reaction is said to be in chemical equilibrium However this equilibrium is said to be dynamic in nature This is because it consists of a forward reaction where the reactants react to give products and reverse
Chemical equilibrium is a dynamic process that consists of a forward reaction in which reactants are converted to products and a reverse reaction in which products are converted to reactants At equilibrium the
Printables for free include a vast collection of printable documents that can be downloaded online at no cost. They are available in a variety of styles, from worksheets to templates, coloring pages and much more. The benefit of Why Is Chemical Equilibrium Described As A Dynamic Process is their flexibility and accessibility.
More of Why Is Chemical Equilibrium Described As A Dynamic Process
13 1 Chemical Equilibria Chemistry 112 Chapters 12 17 Of OpenStax
13 1 Chemical Equilibria Chemistry 112 Chapters 12 17 Of OpenStax
Dynamic equilibrium can be defined as the steady state of a reversible reaction occurring in a closed system Both the forward and reverse reaction proceeds in the opposite direction with equal rates
Dynamic equilibrium Dynamic equilibrium refers to both forward and reverse reactions occurring simultaneously at the same rate while the amount of reactants and products remains
Why Is Chemical Equilibrium Described As A Dynamic Process have gained a lot of popularity for several compelling reasons:
-
Cost-Effective: They eliminate the necessity of purchasing physical copies or expensive software.
-
Modifications: It is possible to tailor printed materials to meet your requirements for invitations, whether that's creating them to organize your schedule or even decorating your house.
-
Education Value Downloads of educational content for free offer a wide range of educational content for learners from all ages, making them a valuable aid for parents as well as educators.
-
It's easy: Fast access various designs and templates cuts down on time and efforts.
Where to Find more Why Is Chemical Equilibrium Described As A Dynamic Process
Chemical Equilibrium Types Conditions Examples And Importance

Chemical Equilibrium Types Conditions Examples And Importance
Dynamic equilibrium can also be established in physical systems for example in a bottle of ethanol Some liquid ethanol will evaporate and some ethanol vapour will condense
Chemical equilibrium is a state where the forward and reverse chemical reactions happen at the same rate so the amounts of reactants and products remain constant It s a
Since we've got your interest in printables for free, let's explore where you can locate these hidden treasures:
1. Online Repositories
- Websites such as Pinterest, Canva, and Etsy have a large selection of Why Is Chemical Equilibrium Described As A Dynamic Process suitable for many uses.
- Explore categories such as decorations for the home, education and organization, and crafts.
2. Educational Platforms
- Educational websites and forums typically offer free worksheets and worksheets for printing or flashcards as well as learning tools.
- It is ideal for teachers, parents, and students seeking supplemental resources.
3. Creative Blogs
- Many bloggers share their imaginative designs and templates for free.
- The blogs are a vast selection of subjects, that range from DIY projects to planning a party.
Maximizing Why Is Chemical Equilibrium Described As A Dynamic Process
Here are some innovative ways create the maximum value of Why Is Chemical Equilibrium Described As A Dynamic Process:
1. Home Decor
- Print and frame gorgeous images, quotes, or decorations for the holidays to beautify your living spaces.
2. Education
- Use these printable worksheets free of charge to aid in learning at your home (or in the learning environment).
3. Event Planning
- Design invitations and banners and decorations for special events like weddings and birthdays.
4. Organization
- Make sure you are organized with printable calendars as well as to-do lists and meal planners.
Conclusion
Why Is Chemical Equilibrium Described As A Dynamic Process are a treasure trove of useful and creative resources which cater to a wide range of needs and interests. Their accessibility and versatility make these printables a useful addition to any professional or personal life. Explore the world of Why Is Chemical Equilibrium Described As A Dynamic Process now and unlock new possibilities!
Frequently Asked Questions (FAQs)
-
Are printables actually free?
- Yes you can! You can print and download these materials for free.
-
Does it allow me to use free templates for commercial use?
- It's determined by the specific terms of use. Always read the guidelines of the creator before using printables for commercial projects.
-
Are there any copyright concerns when using printables that are free?
- Certain printables may be subject to restrictions in use. Check the terms and conditions offered by the creator.
-
How can I print Why Is Chemical Equilibrium Described As A Dynamic Process?
- Print them at home with a printer or visit a local print shop to purchase premium prints.
-
What program must I use to open printables free of charge?
- Many printables are offered in the PDF format, and can be opened using free programs like Adobe Reader.
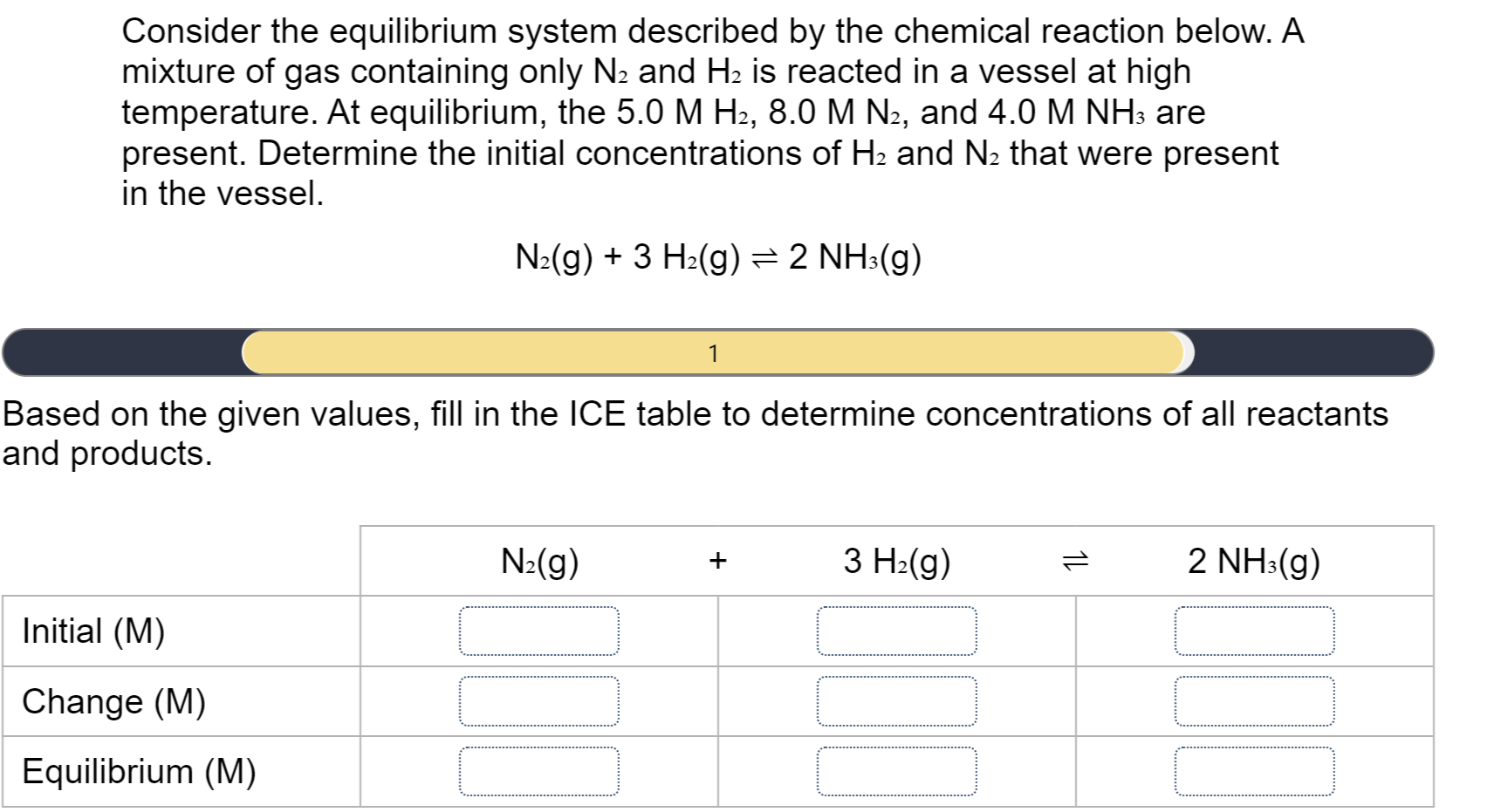
Answered Consider The Equilibrium System Bartleby
Chemical Equilibrum Passnownow

Check more sample of Why Is Chemical Equilibrium Described As A Dynamic Process below
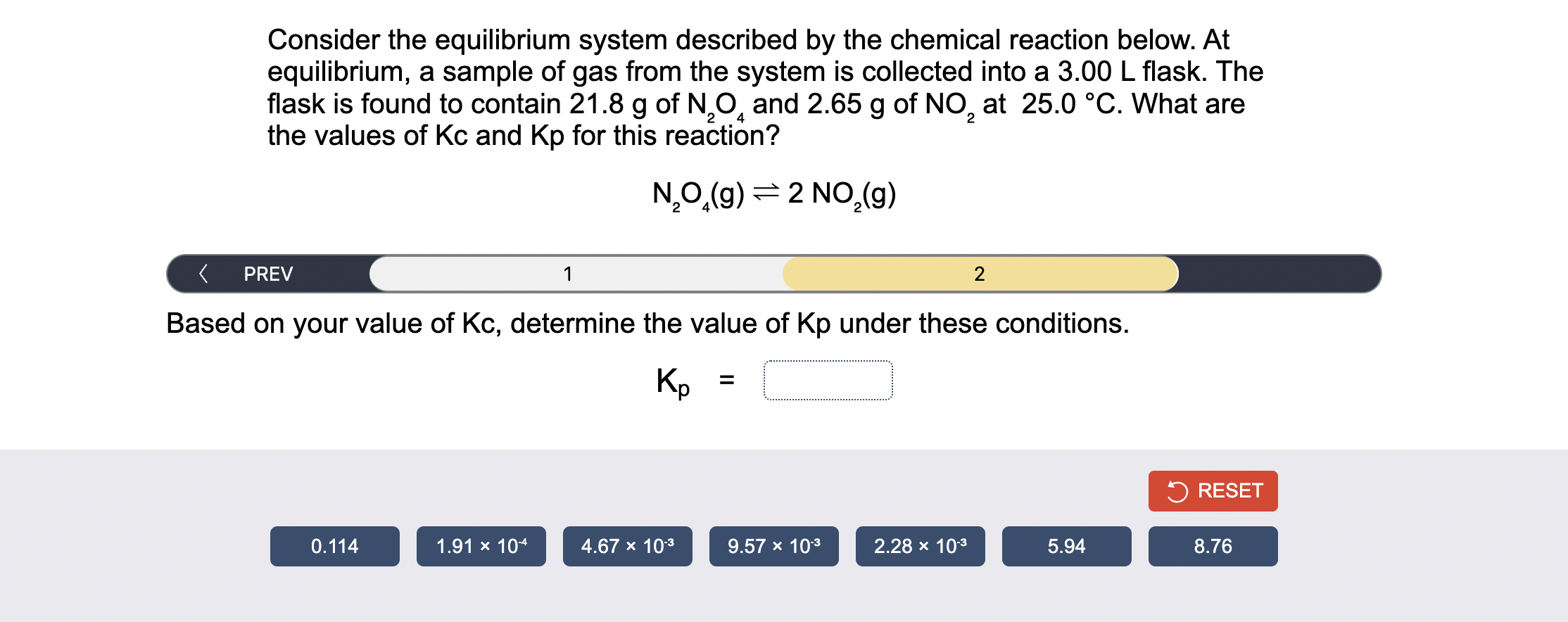
Solved Consider The Equilibrium System Described By The Chegg
Why Is Dynamic Used To Describe Chemical Equilibrium

Solved Please Help Consider The Equilibrium System Described By The
When Do Reactions Reach Chemical Equilibrium And Why Does Chemical

Chemical Equilibrium 838 Plays Quizizz
Solved Consider The Equilibrium System Described By The Chegg

https://chem.libretexts.org › Bookshelves › …
Chemical equilibrium is a dynamic process that consists of a forward reaction in which reactants are converted to products and a reverse reaction in which products are converted to reactants At equilibrium the
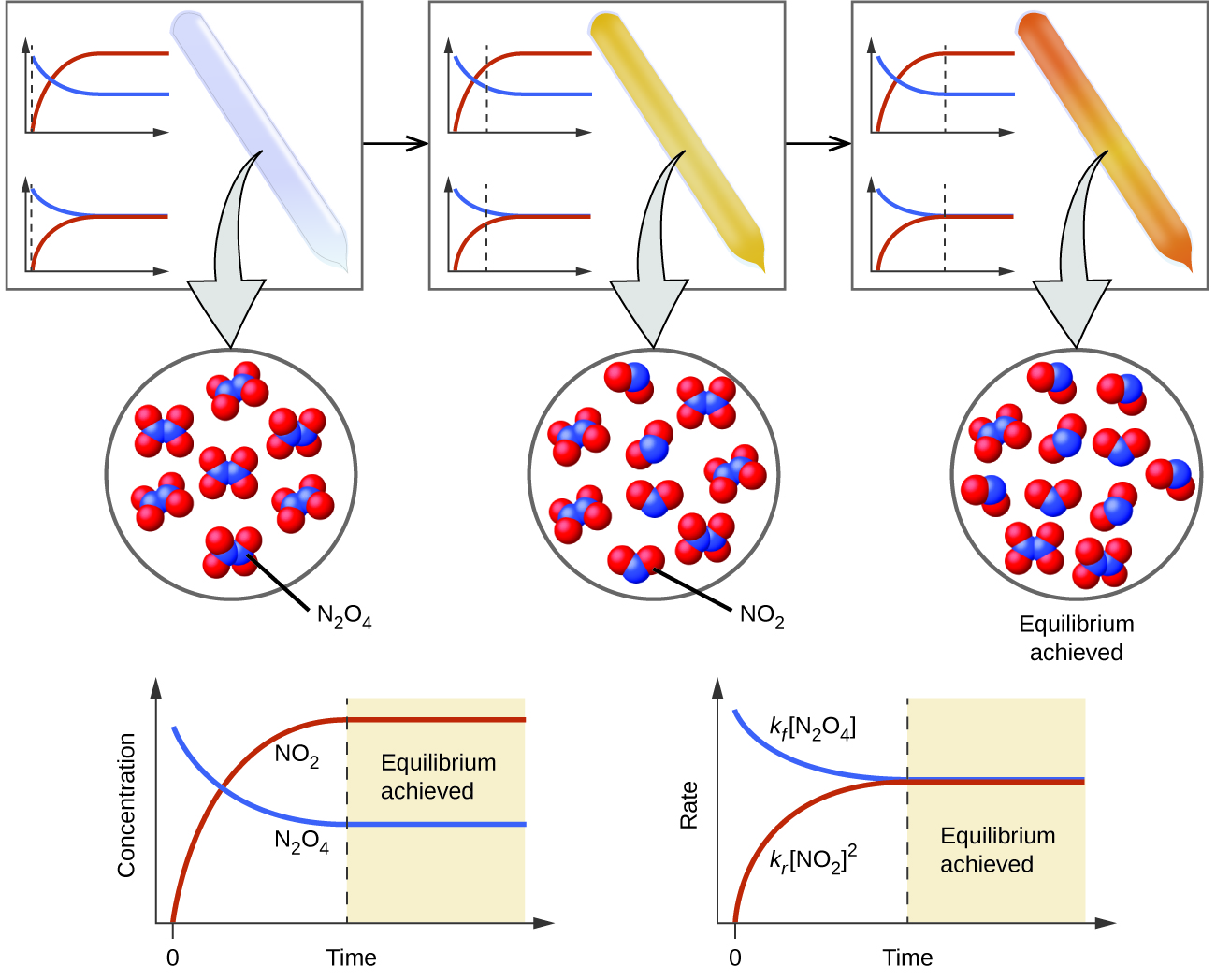
https://chem.libretexts.org › Bookshelves...
Chemical equilibrium occurs when the number of particles becoming products is equal to the number of particles becoming reactants A dynamic equilibrium is a state where the rate of the forward reaction is equal
Chemical equilibrium is a dynamic process that consists of a forward reaction in which reactants are converted to products and a reverse reaction in which products are converted to reactants At equilibrium the
Chemical equilibrium occurs when the number of particles becoming products is equal to the number of particles becoming reactants A dynamic equilibrium is a state where the rate of the forward reaction is equal

When Do Reactions Reach Chemical Equilibrium And Why Does Chemical

Why Is Dynamic Used To Describe Chemical Equilibrium
Chemical Equilibrium 838 Plays Quizizz
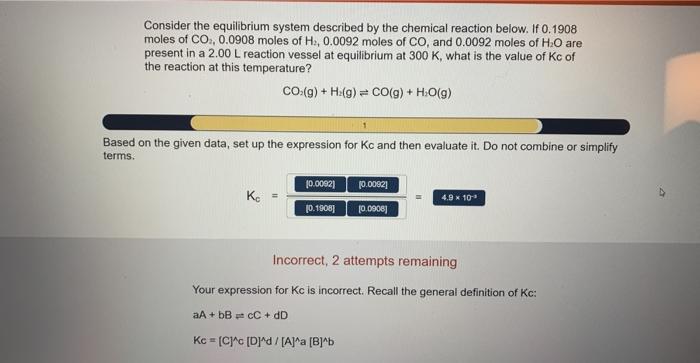
Solved Consider The Equilibrium System Described By The Chegg
Chemical Equilibrium What Are The Characteristics Of Equilibrium
Solved Consider The Equilibrium System Described By The

Solved Consider The Equilibrium System Described By The
Solved Consider The Equilibrium System Described By The Chegg