In the digital age, when screens dominate our lives however, the attraction of tangible printed material hasn't diminished. In the case of educational materials, creative projects, or just adding the personal touch to your home, printables for free are a great source. We'll dive to the depths of "Why Is Chemical Equilibrium Dynamic," exploring the benefits of them, where to find them and how they can be used to enhance different aspects of your life.
Get Latest Why Is Chemical Equilibrium Dynamic Below
Why Is Chemical Equilibrium Dynamic
Why Is Chemical Equilibrium Dynamic - Why Is Chemical Equilibrium Dynamic, Why Is Chemical Equilibrium Described As Dynamic, Why Chemical Equilibrium Is Dynamic Class 10, Why Chemical Equilibrium Is Dynamic In Nature Explain With Example, Why Chemical Equilibrium Is Dynamic Class 10 Answer, Equilibrium In Chemical Processes Dynamic Equilibrium, Why Is Chemical Equilibrium Considered To Be A Dynamic State, Why Is Chemical Equilibrium Referred To As Dynamic Equilibrium, Why Is A Reaction At Chemical Equilibrium Described As Dynamic, Why Is Chemical Equilibrium Considered Dynamic
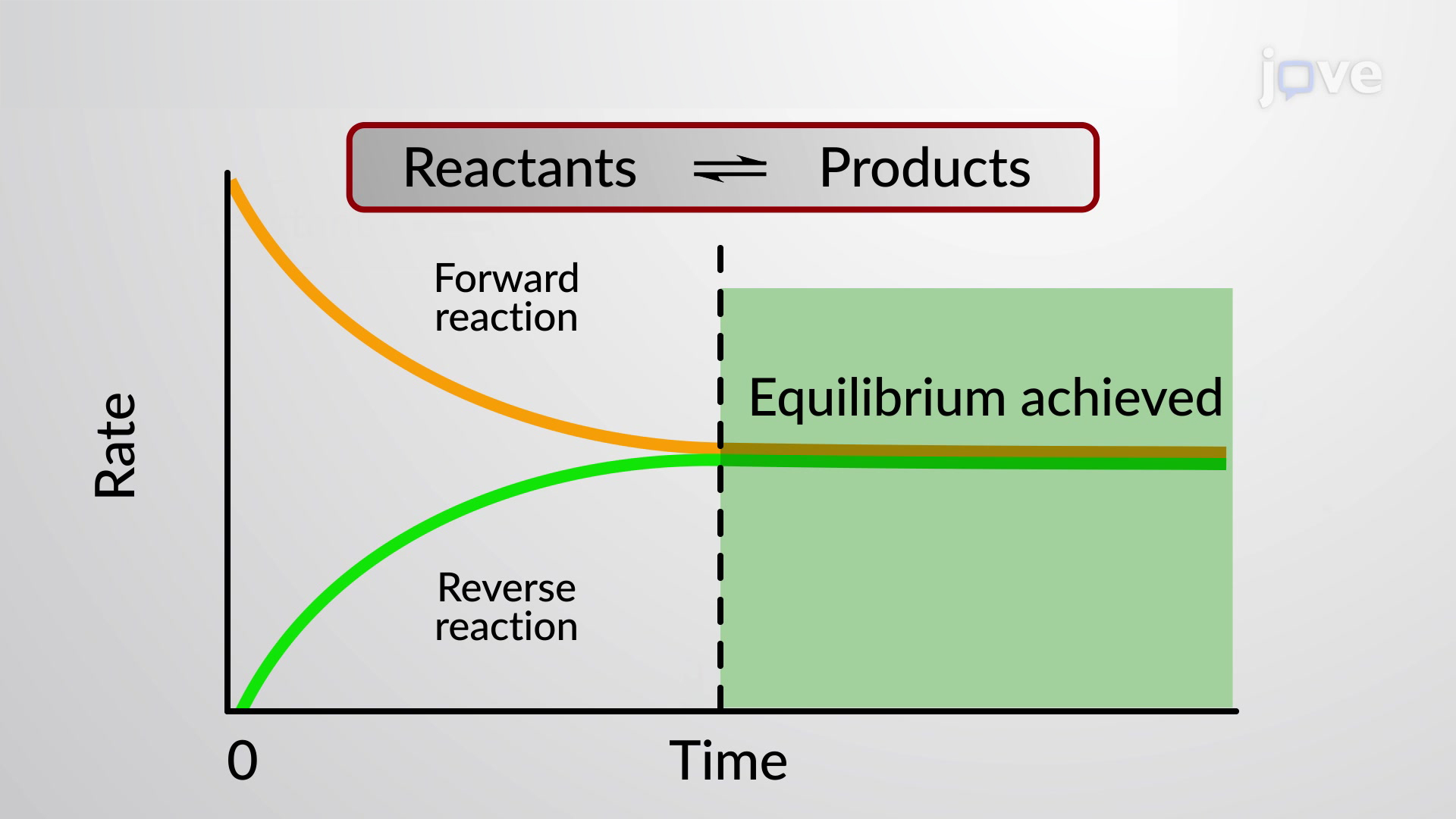
Equilibrium is macroscopically static but is microscopically dynamic To further illustrate the dynamic character of chemical equilibrium suppose that we now change the composition of the system previously at equilibrium by adding some C or withdrawing some A thus changing their active masses The reverse rate will
Chemical equilibrium occurs when the number of particles becoming products is equal to the number of particles becoming reactants A dynamic equilibrium is a state where the rate of the forward reaction is equal to the rate of the reverse reaction
Printables for free cover a broad assortment of printable materials that are accessible online for free cost. The resources are offered in a variety types, such as worksheets templates, coloring pages and many more. The benefit of Why Is Chemical Equilibrium Dynamic is in their variety and accessibility.
More of Why Is Chemical Equilibrium Dynamic
Chemistry A Level Revision Equilibrium
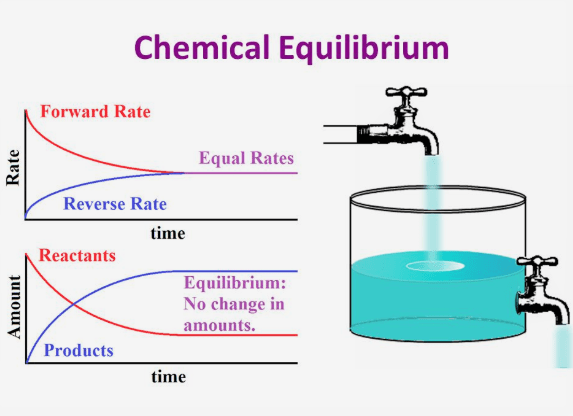
Chemistry A Level Revision Equilibrium
Dynamic equilibrium is an important concept in chemistry But what is dynamic equilibrium exactly How can something be dynamic but also at equilibrium Keep reading to learn the best dynamic equilibrium definition common dynamic equilibrium examples and how dynamic and static equilibrium may look the same but are in fact
Why is Chemical Equilibrium called Dynamic Equilibrium During equilibrium the forward reaction rate becomes equal to the backward reaction rate At this time the number of reactant molecules transforming into products and the number of product molecules converting into reactants are the same
Why Is Chemical Equilibrium Dynamic have garnered immense popularity due to a myriad of compelling factors:
-
Cost-Efficiency: They eliminate the need to purchase physical copies or expensive software.
-
Flexible: This allows you to modify printables to fit your particular needs in designing invitations, organizing your schedule, or decorating your home.
-
Education Value Free educational printables offer a wide range of educational content for learners of all ages, which makes them a great resource for educators and parents.
-
The convenience of Quick access to an array of designs and templates cuts down on time and efforts.
Where to Find more Why Is Chemical Equilibrium Dynamic
What Is Thermodynamic Equilibrium With Best Examples

What Is Thermodynamic Equilibrium With Best Examples
Part of Chemistry Chemistry in society Remove from My Bitesize Dynamic equilibrium Many chemical reactions are reversible In these reactions there is both a forward reaction where reactants
Dynamic equilibrium is the point at which a chemical reaction continues to proceed both forward and in reverse but there is no net change in the amount of reactants and products
After we've peaked your interest in printables for free We'll take a look around to see where the hidden gems:
1. Online Repositories
- Websites such as Pinterest, Canva, and Etsy provide a wide selection of Why Is Chemical Equilibrium Dynamic designed for a variety uses.
- Explore categories such as interior decor, education, the arts, and more.
2. Educational Platforms
- Educational websites and forums frequently offer free worksheets and worksheets for printing Flashcards, worksheets, and other educational materials.
- Ideal for parents, teachers and students looking for extra resources.
3. Creative Blogs
- Many bloggers share their imaginative designs and templates free of charge.
- The blogs are a vast range of topics, starting from DIY projects to planning a party.
Maximizing Why Is Chemical Equilibrium Dynamic
Here are some creative ways how you could make the most of printables for free:
1. Home Decor
- Print and frame stunning artwork, quotes, or other seasonal decorations to fill your living spaces.
2. Education
- Use printable worksheets from the internet to help reinforce your learning at home, or even in the classroom.
3. Event Planning
- Design invitations, banners, and decorations for special occasions like birthdays and weddings.
4. Organization
- Get organized with printable calendars including to-do checklists, daily lists, and meal planners.
Conclusion
Why Is Chemical Equilibrium Dynamic are an abundance of fun and practical tools which cater to a wide range of needs and passions. Their availability and versatility make them a fantastic addition to every aspect of your life, both professional and personal. Explore the endless world of Why Is Chemical Equilibrium Dynamic today to discover new possibilities!
Frequently Asked Questions (FAQs)
-
Are Why Is Chemical Equilibrium Dynamic truly gratis?
- Yes you can! You can download and print these files for free.
-
Can I download free printables for commercial use?
- It's determined by the specific rules of usage. Always verify the guidelines of the creator before utilizing printables for commercial projects.
-
Do you have any copyright violations with Why Is Chemical Equilibrium Dynamic?
- Some printables may come with restrictions on use. You should read the terms and regulations provided by the designer.
-
How can I print printables for free?
- Print them at home using printing equipment or visit an in-store print shop to get superior prints.
-
What program is required to open printables free of charge?
- The majority of printed documents are in the format of PDF, which is open with no cost software, such as Adobe Reader.
Chemical Equilibrium 826 Plays Quizizz
Why Is Dynamic Used To Describe Chemical Equilibrium

Check more sample of Why Is Chemical Equilibrium Dynamic below
Dynamic Equilibrium Definition Important Examples
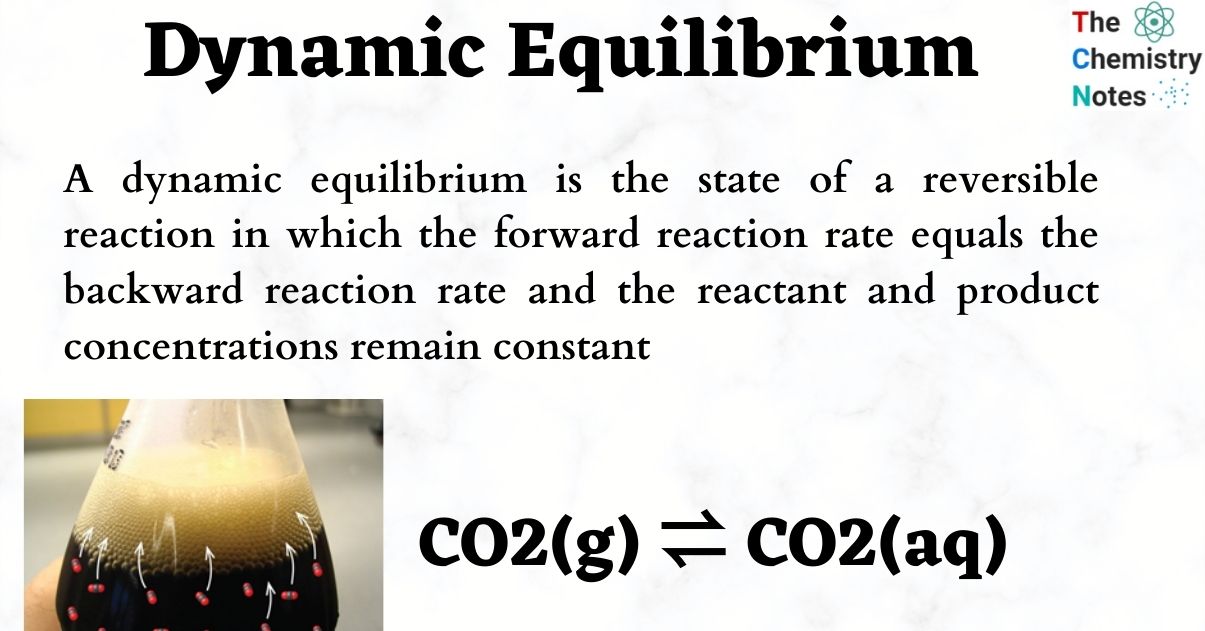
Chemical Equilibrium What Are The Characteristics Of Equilibrium
Equilibrium Made Easy How To Solve Chemical Equilibrium Problems YouTube

Chemical Equilibrium
Chemical Equilibrium Mindmeister Mind Map Gambaran
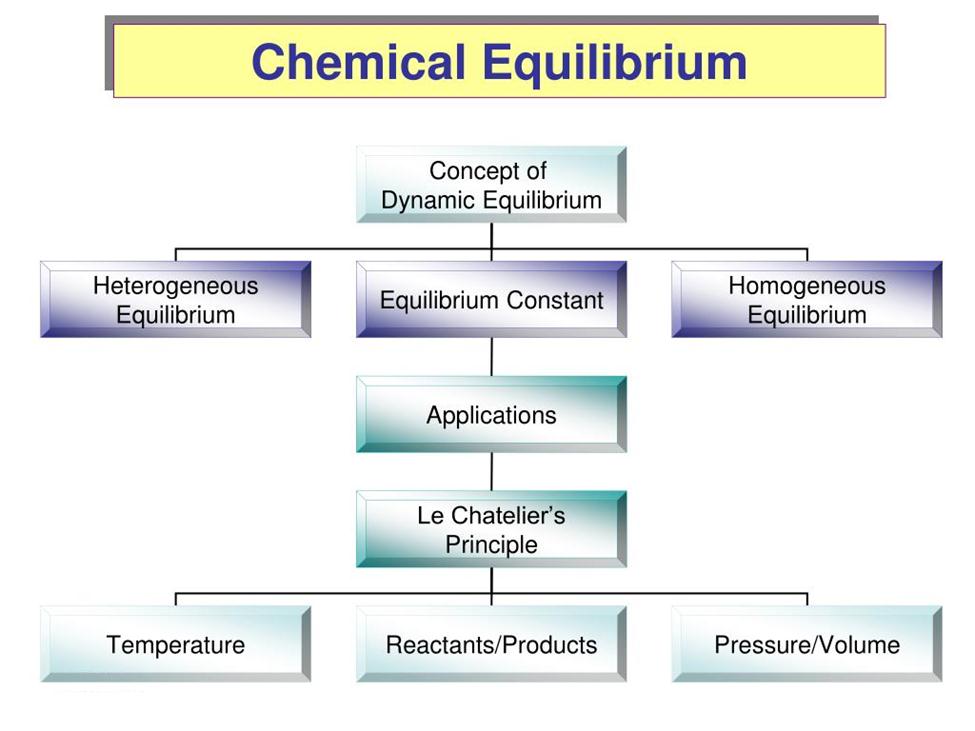
Le Chateliers Principle Steps Of Chemistry 2022
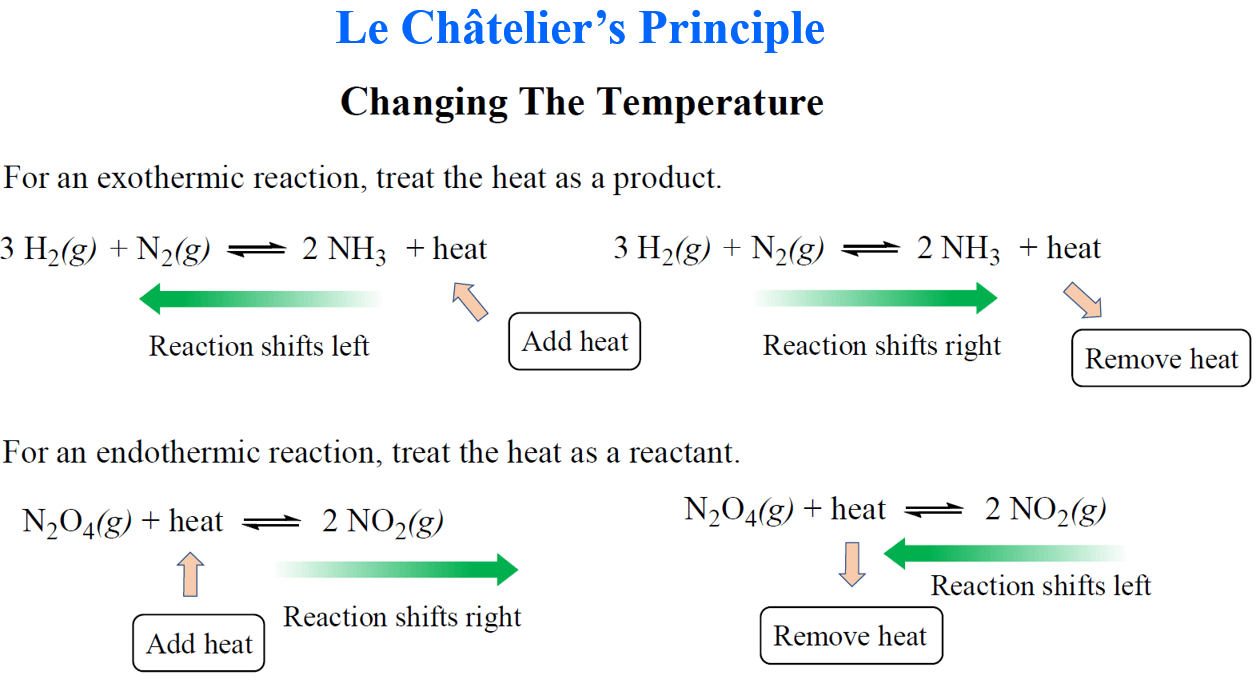

https://chem.libretexts.org/Bookshelves...
Chemical equilibrium occurs when the number of particles becoming products is equal to the number of particles becoming reactants A dynamic equilibrium is a state where the rate of the forward reaction is equal to the rate of the reverse reaction

https://chem.libretexts.org/Bookshelves/General...
Chemical equilibrium is a dynamic process that consists of a forward reaction in which reactants are converted to products and a reverse reaction in which products are converted to reactants At equilibrium the forward and reverse reactions proceed at equal rates
Chemical equilibrium occurs when the number of particles becoming products is equal to the number of particles becoming reactants A dynamic equilibrium is a state where the rate of the forward reaction is equal to the rate of the reverse reaction
Chemical equilibrium is a dynamic process that consists of a forward reaction in which reactants are converted to products and a reverse reaction in which products are converted to reactants At equilibrium the forward and reverse reactions proceed at equal rates

Chemical Equilibrium

Chemical Equilibrium What Are The Characteristics Of Equilibrium

Chemical Equilibrium Mindmeister Mind Map Gambaran

Le Chateliers Principle Steps Of Chemistry 2022

Difference Between Static And Dynamic Equilibrium Equilibrium
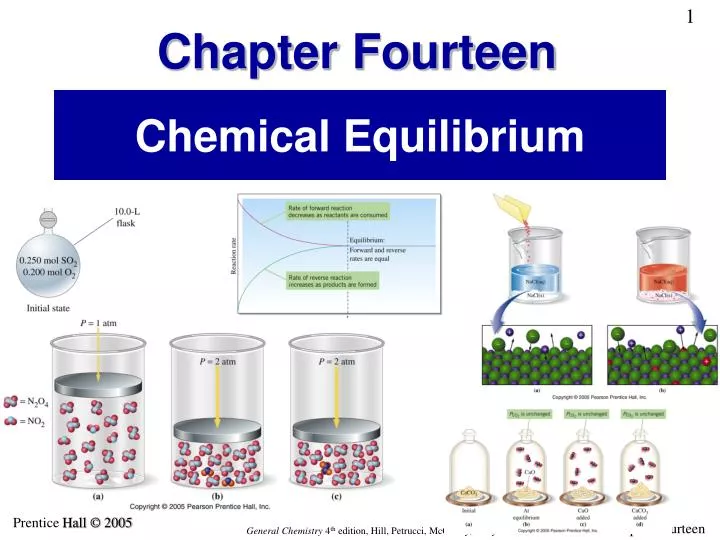
PPT Chemical Equilibrium PowerPoint Presentation ID 3889861

PPT Chemical Equilibrium PowerPoint Presentation ID 3889861
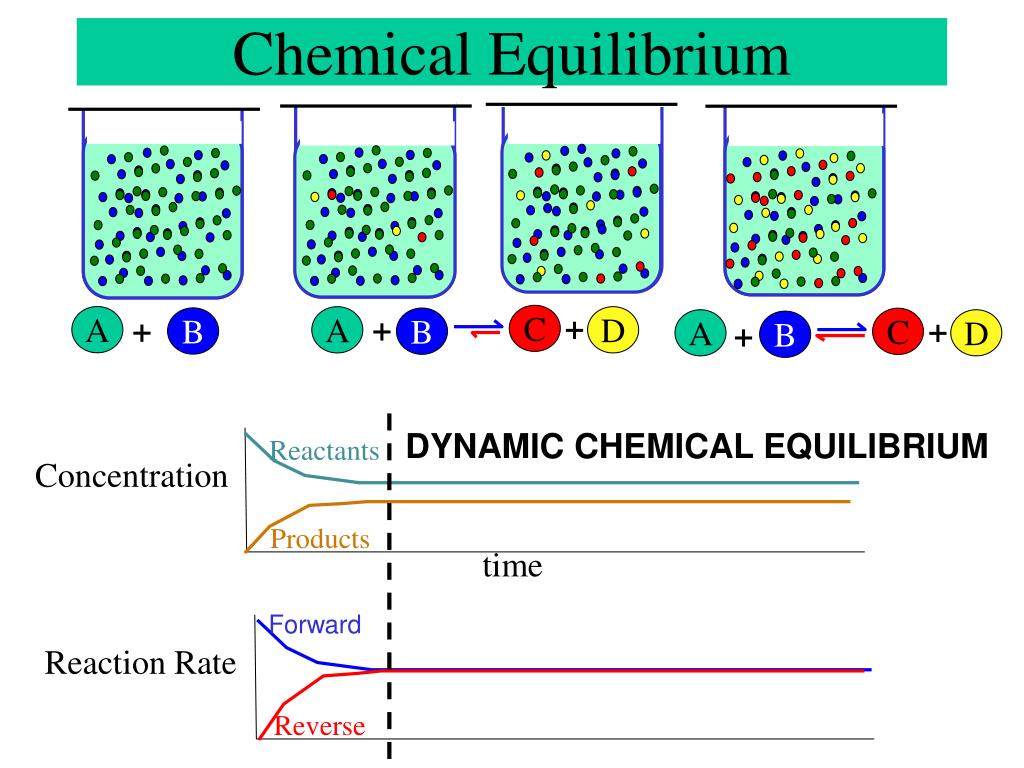
PPT Chemical Equilibrium PowerPoint Presentation Free Download ID