Today, where screens rule our lives and the appeal of physical, printed materials hasn't diminished. Be it for educational use such as creative projects or simply to add an individual touch to the area, What Is Static Equilibrium In Chemistry can be an excellent source. The following article is a dive through the vast world of "What Is Static Equilibrium In Chemistry," exploring the different types of printables, where to find them and how they can improve various aspects of your life.
Get Latest What Is Static Equilibrium In Chemistry Below
What Is Static Equilibrium In Chemistry
What Is Static Equilibrium In Chemistry - What Is Static Equilibrium In Chemistry, What Is Static Equilibrium In Chemistry With Example, What Is Static Equilibrium In Chemistry Class 11, What Is Dynamic Equilibrium In Chemistry Gcse, What Is Meant By Dynamic Equilibrium In Chemistry, What Is Dynamic Equilibrium Reactions Chemistry Fuseschool, What Is Dynamic Equilibrium A Level Chemistry, What Is The Difference Between Static And Dynamic Equilibrium In Chemistry, Define Static Equilibrium In Chemistry, What Is Static Equilibrium
A reaction is at equilibrium when the amounts of reactants or products no longer change Chemical equilibrium is a dynamic process meaning the rate of formation of products by the forward reaction is equal to the rate at which the products re form reactants by the reverse reaction
Equilibrium is macroscopically static but is microscopically dynamic To further illustrate the dynamic character of chemical equilibrium suppose that we now change the composition of the system previously at equilibrium by adding some C or withdrawing some A thus changing their active masses
Printables for free cover a broad assortment of printable, downloadable material that is available online at no cost. They are available in numerous types, such as worksheets coloring pages, templates and much more. The great thing about What Is Static Equilibrium In Chemistry is in their versatility and accessibility.
More of What Is Static Equilibrium In Chemistry
Chemical Equilibrium I Types Of Equilibrium Equilibrium Constant

Chemical Equilibrium I Types Of Equilibrium Equilibrium Constant
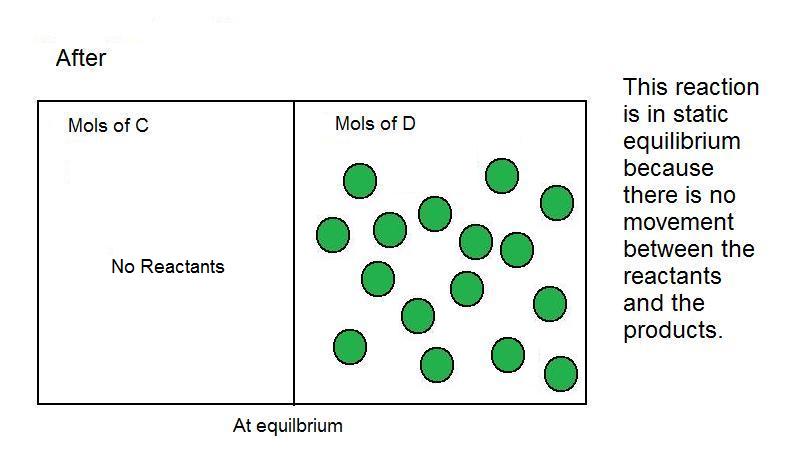
Dynamic equilibrium is an equilibrium where reactants are converted to products and products are converted to reactants at an equal and constant rate Static equilibrium is an equilibrium that occurs when all particles in the reaction are at rest and there is no motion between reactants and products
Dynamic equilibrium is the steady state of a reversible reaction where the rate of the forward reaction is the same as the reaction rate in the backward direction Static equilibrium also known as mechanical equilibrium means the reaction has stopped In other words the system is at rest
What Is Static Equilibrium In Chemistry have risen to immense popularity due to a myriad of compelling factors:
-
Cost-Effective: They eliminate the necessity of purchasing physical copies or expensive software.
-
Customization: The Customization feature lets you tailor printables to your specific needs whether you're designing invitations, organizing your schedule, or even decorating your home.
-
Educational Value These What Is Static Equilibrium In Chemistry can be used by students of all ages, which makes these printables a powerful device for teachers and parents.
-
Affordability: Instant access to a variety of designs and templates saves time and effort.
Where to Find more What Is Static Equilibrium In Chemistry
Chemical Equilibrium What Are The Characteristics Of Equilibrium
Chemical Equilibrium What Are The Characteristics Of Equilibrium
Static equilibrium refers to a condition when the reaction occurring in the system comes to a halt Therefore the motion between reactants and the products ceases leading to no exchange between reactants and products Here the equilibrium is attained once all the limiting reagents are used up
Static equilibrium is an equilibrium that occurs when all particles in the reaction are at rest and there is no motion between reactants and products Dynamic equilibrium is an equilibrium that occurs when the rate of formation of product and the rate of decay of product back to reactant is same
We've now piqued your interest in printables for free Let's take a look at where the hidden treasures:
1. Online Repositories
- Websites like Pinterest, Canva, and Etsy offer a huge selection of What Is Static Equilibrium In Chemistry suitable for many motives.
- Explore categories like interior decor, education, organizing, and crafts.
2. Educational Platforms
- Educational websites and forums usually provide worksheets that can be printed for free with flashcards and other teaching tools.
- Ideal for teachers, parents and students who are in need of supplementary resources.
3. Creative Blogs
- Many bloggers share their creative designs as well as templates for free.
- The blogs are a vast range of interests, including DIY projects to planning a party.
Maximizing What Is Static Equilibrium In Chemistry
Here are some unique ways for you to get the best use of printables for free:
1. Home Decor
- Print and frame gorgeous images, quotes, as well as seasonal decorations, to embellish your living spaces.
2. Education
- Use printable worksheets from the internet to aid in learning at your home also in the classes.
3. Event Planning
- Design invitations, banners, and decorations for special events like birthdays and weddings.
4. Organization
- Be organized by using printable calendars or to-do lists. meal planners.
Conclusion
What Is Static Equilibrium In Chemistry are a treasure trove filled with creative and practical information that meet a variety of needs and needs and. Their availability and versatility make them a fantastic addition to your professional and personal life. Explore the world of What Is Static Equilibrium In Chemistry now and discover new possibilities!
Frequently Asked Questions (FAQs)
-
Are printables available for download really for free?
- Yes they are! You can print and download these files for free.
-
Can I use the free printouts for commercial usage?
- It depends on the specific terms of use. Always verify the guidelines of the creator prior to utilizing the templates for commercial projects.
-
Do you have any copyright concerns with What Is Static Equilibrium In Chemistry?
- Some printables could have limitations on usage. Always read the terms and condition of use as provided by the creator.
-
How can I print What Is Static Equilibrium In Chemistry?
- You can print them at home using your printer or visit any local print store for higher quality prints.
-
What software must I use to open printables for free?
- The majority of printed documents are with PDF formats, which can be opened with free software like Adobe Reader.
Chapter 12 Static Equilibrium
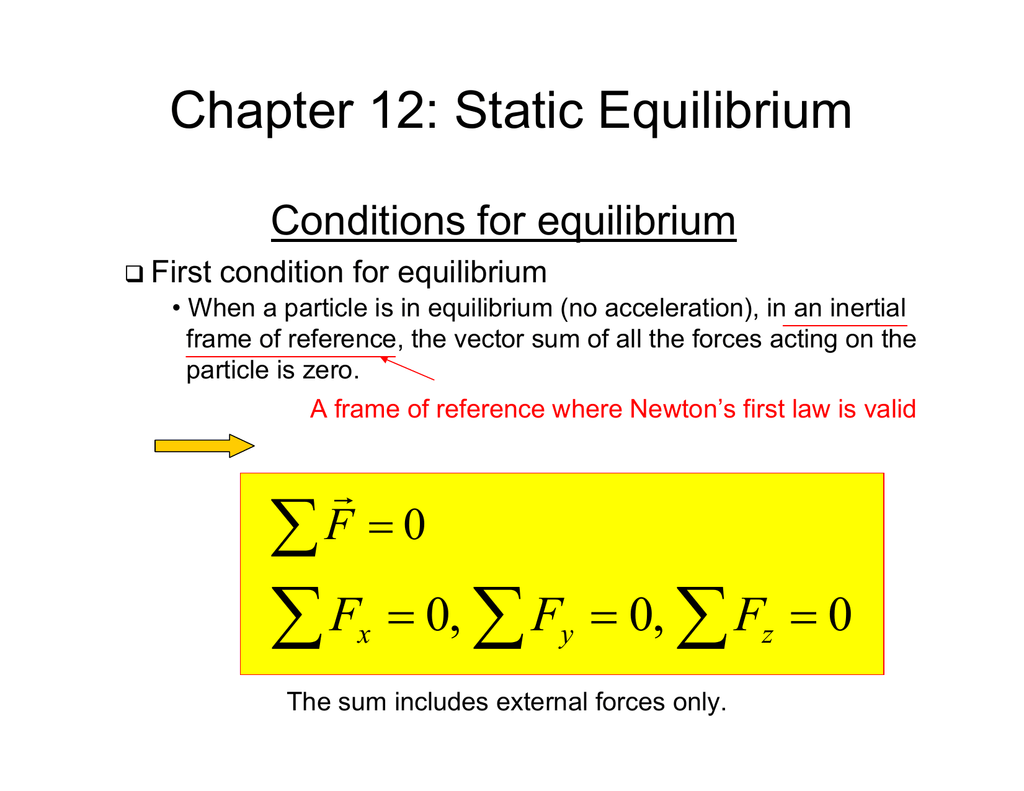
Difference Between Static And Dynamic Equilibrium Equilibrium

Check more sample of What Is Static Equilibrium In Chemistry below
Chemical Equilibrium 809 Plays Quizizz
7 Important Difference Between Static And Dynamic Equilibrium With

Chemical Equilibria Chemistry
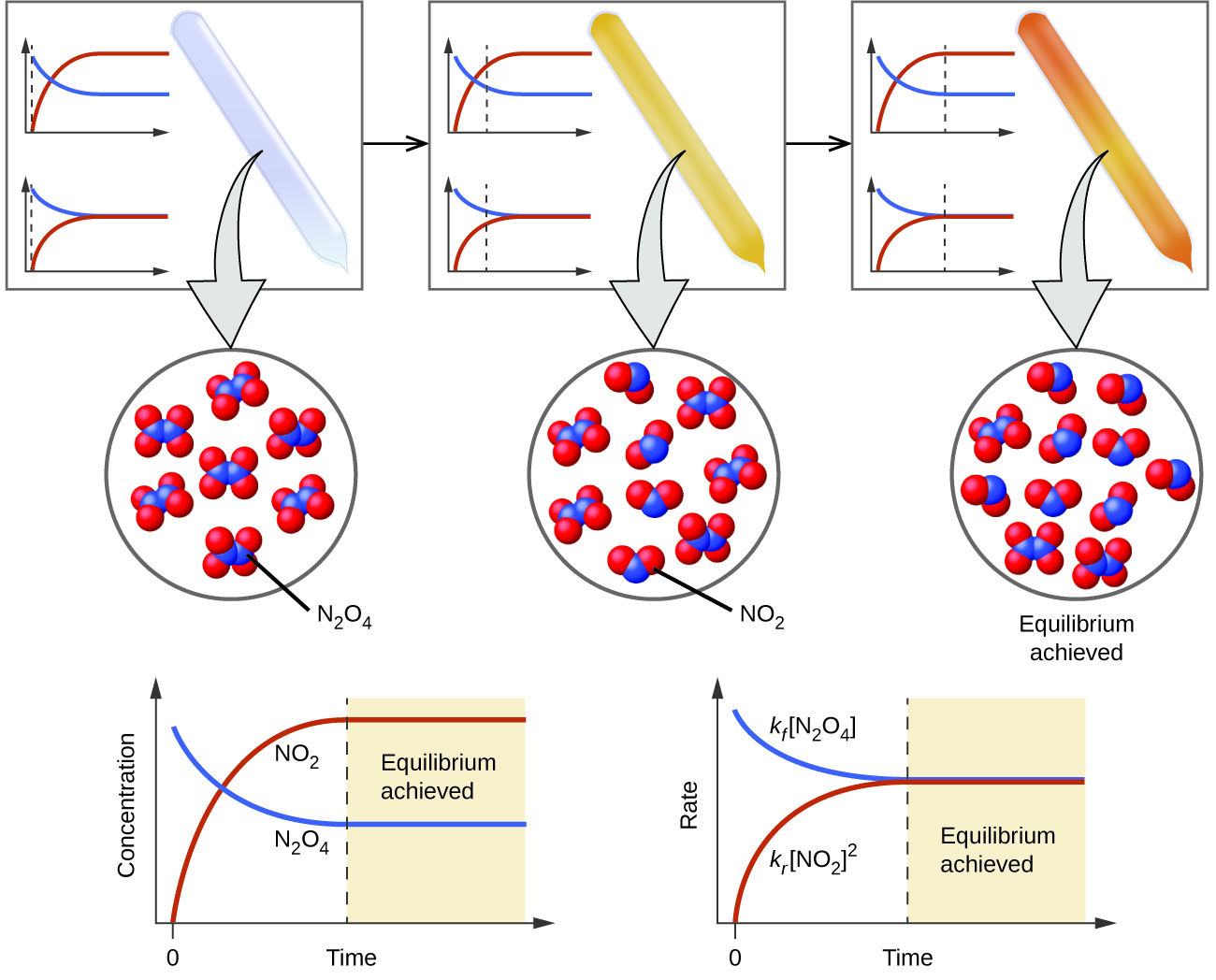
PPT Chemical Equilibrium PowerPoint Presentation ID 3889861
Dynamic Equilibrium Chemistry LibreTexts

Equilibrium Chemical Equilibrium And Physical Equilibrium YouTube


https://chem.libretexts.org/Bookshelves/General...
Equilibrium is macroscopically static but is microscopically dynamic To further illustrate the dynamic character of chemical equilibrium suppose that we now change the composition of the system previously at equilibrium by adding some C or withdrawing some A thus changing their active masses
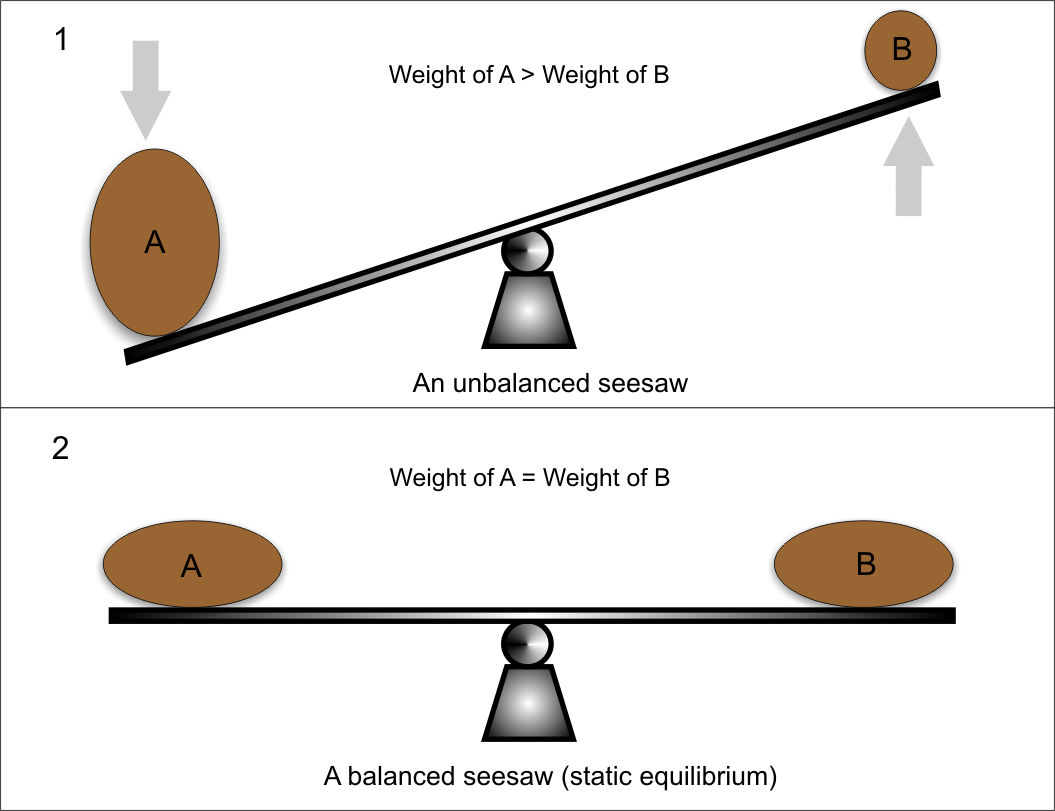
https://byjus.com/jee/dynamic-equilibrium
Static equilibrium refers to a condition where the reaction occurring in a system is completely halted and there exists no movement between the reactants and the products corresponding to the chemical reaction
Equilibrium is macroscopically static but is microscopically dynamic To further illustrate the dynamic character of chemical equilibrium suppose that we now change the composition of the system previously at equilibrium by adding some C or withdrawing some A thus changing their active masses
Static equilibrium refers to a condition where the reaction occurring in a system is completely halted and there exists no movement between the reactants and the products corresponding to the chemical reaction

PPT Chemical Equilibrium PowerPoint Presentation ID 3889861

7 Important Difference Between Static And Dynamic Equilibrium With

Dynamic Equilibrium Chemistry LibreTexts

Equilibrium Chemical Equilibrium And Physical Equilibrium YouTube

Dynamic Chemical Equilibrium Definition Examples Video Lesson
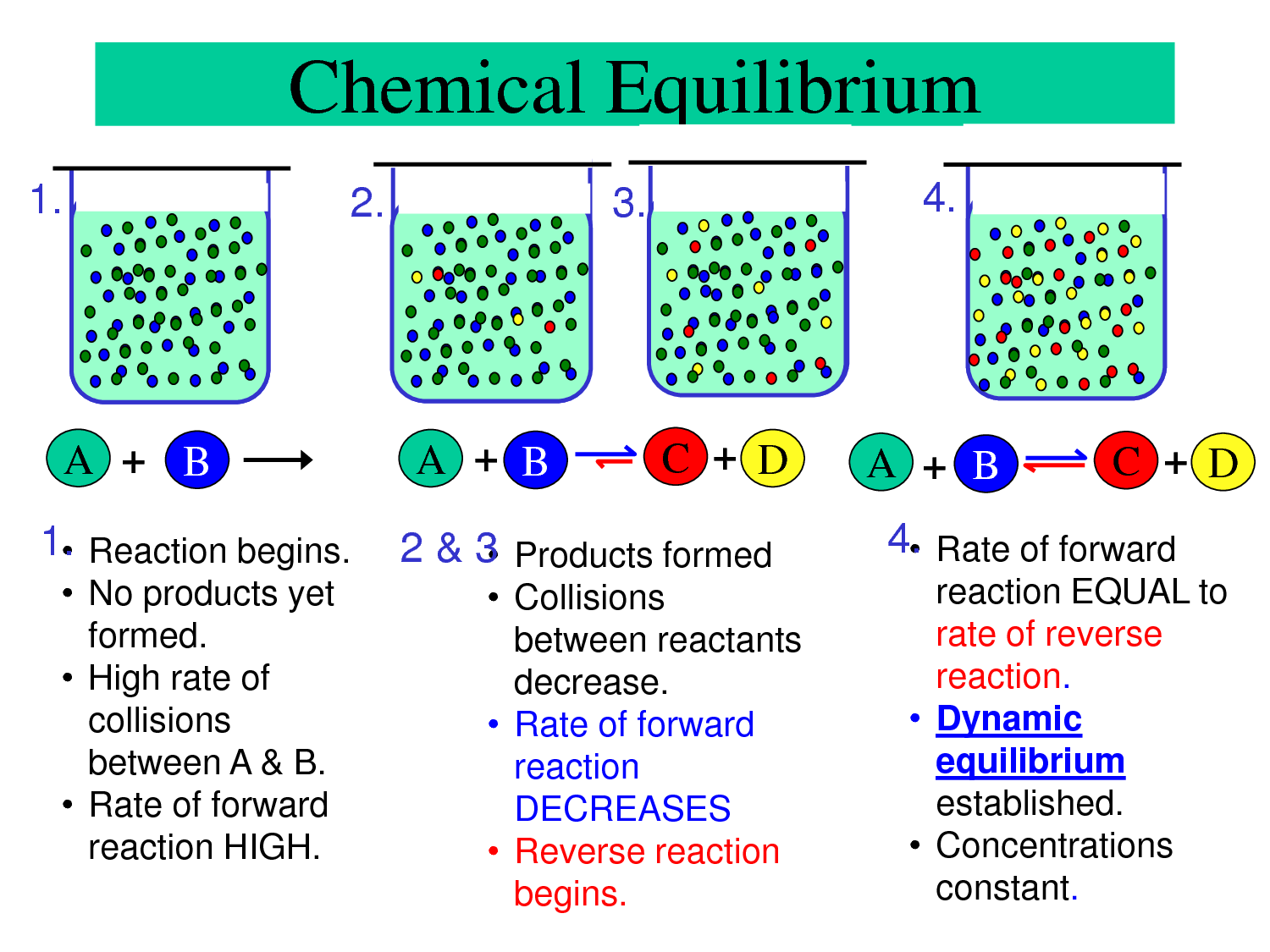
Chemical Equilibrum Passnownow

Chemical Equilibrum Passnownow
Chemical Equilibrium