In this day and age in which screens are the norm however, the attraction of tangible printed items hasn't gone away. For educational purposes in creative or artistic projects, or just adding the personal touch to your area, What Is Dynamic Equilibrium Reactions are now a vital resource. Here, we'll take a dive into the world of "What Is Dynamic Equilibrium Reactions," exploring the benefits of them, where to find them and how they can enhance various aspects of your lives.
Get Latest What Is Dynamic Equilibrium Reactions Below
What Is Dynamic Equilibrium Reactions
What Is Dynamic Equilibrium Reactions - What Is Dynamic Equilibrium Reactions, What Is Dynamic Equilibrium Chemistry, What Is Dynamic Equilibrium Chemistry Gcse, What Is Dynamic Equilibrium Reactions Chemistry Fuseschool, What Is Dynamic Equilibrium Reversible Reaction, What Is Dynamic Equilibrium In Chemistry Class 11, What Is Dynamic Equilibrium In Chemistry Class 10, What Happens During Dynamic Equilibrium
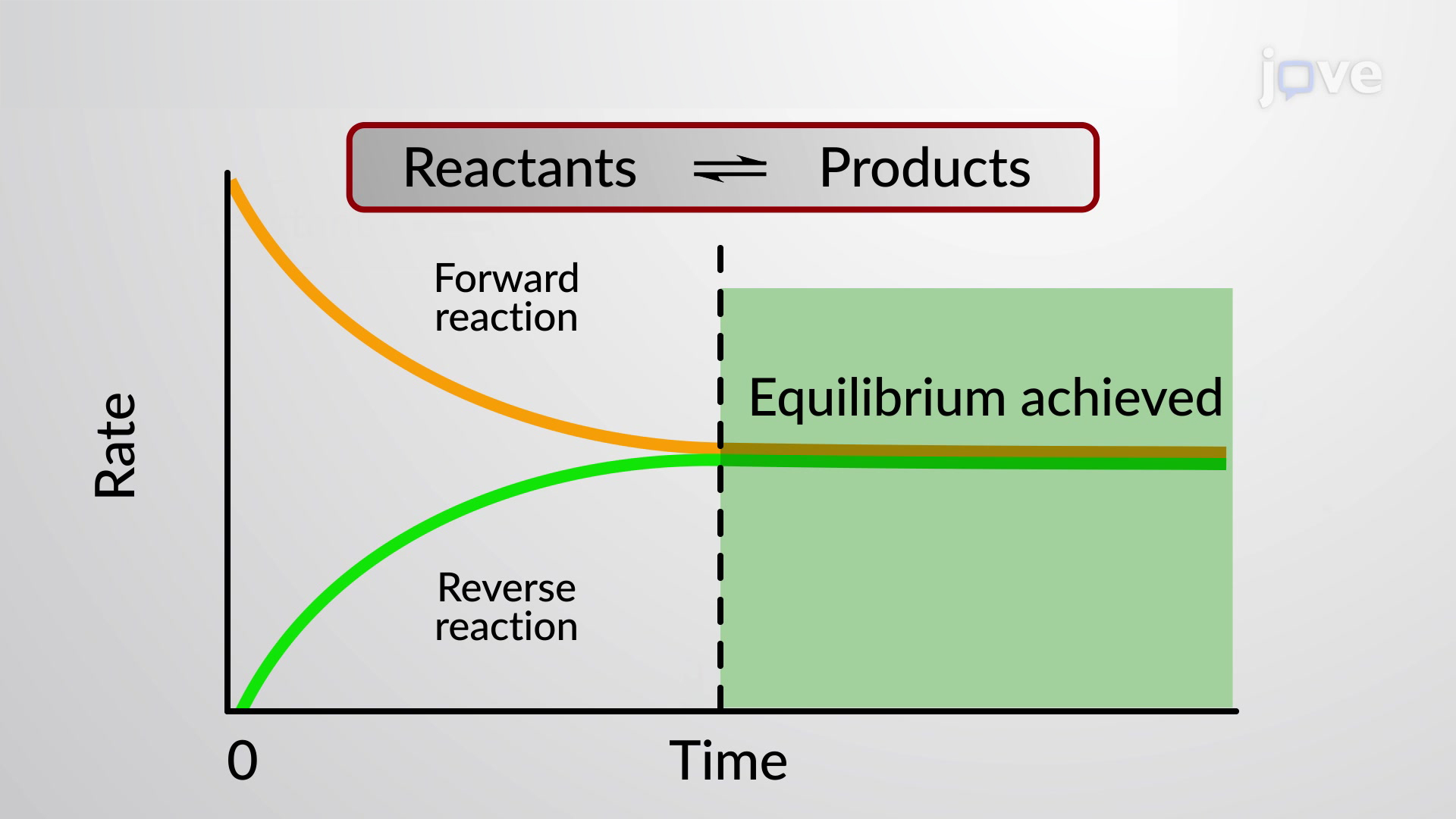
A reaction is at equilibrium when the amounts of reactants or products no longer change Chemical equilibrium is a dynamic process meaning the rate of formation of products by the forward reaction is equal to
Dynamic equilibrium Many chemical reactions are reversible In these reactions there is both a forward reaction where reactants are made into products and a reverse reaction where
What Is Dynamic Equilibrium Reactions provide a diverse range of printable, free materials that are accessible online for free cost. These printables come in different formats, such as worksheets, templates, coloring pages and much more. The attraction of printables that are free is their versatility and accessibility.
More of What Is Dynamic Equilibrium Reactions
18 Unbelievable Facts About Dynamic Equilibrium Facts
18 Unbelievable Facts About Dynamic Equilibrium Facts
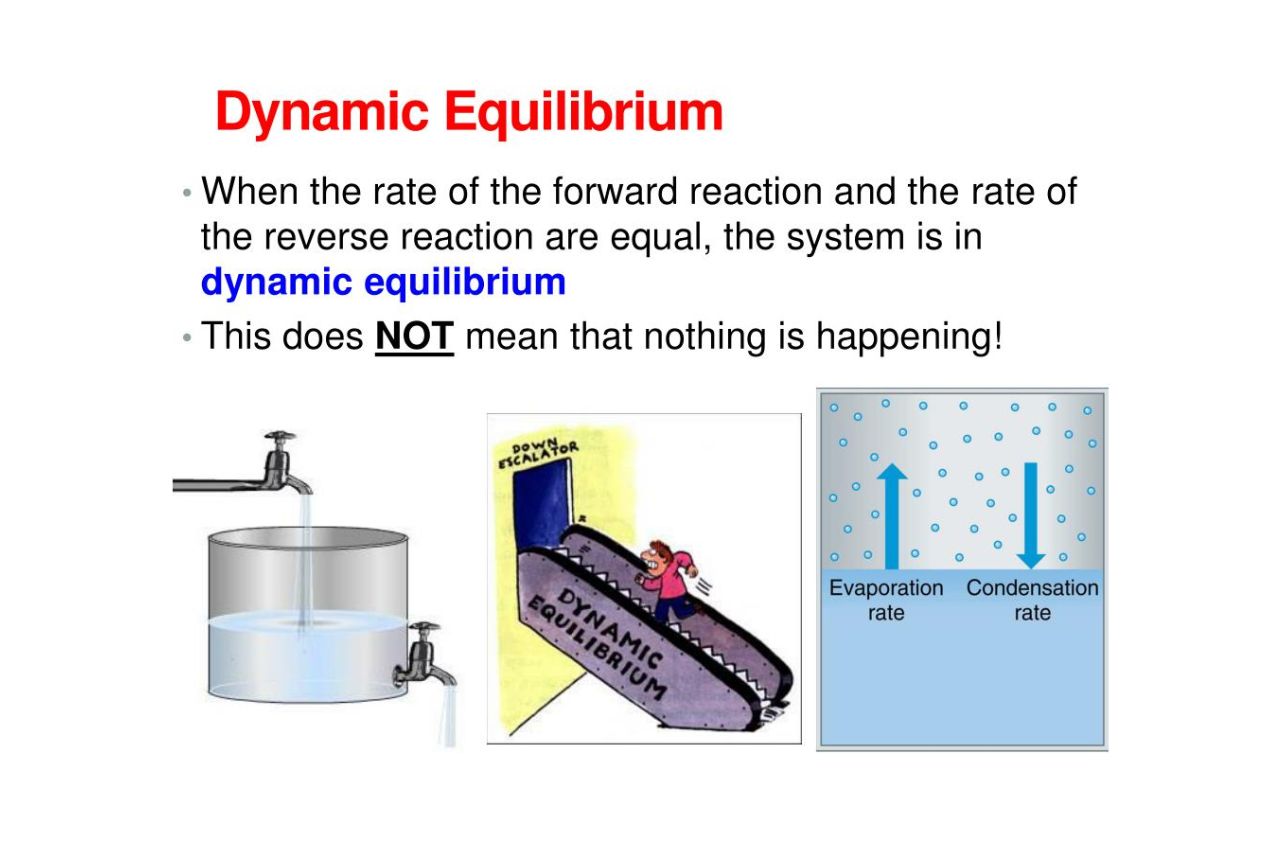
In chemistry a dynamic equilibrium exists once a reversible reaction occurs Substances transition between the reactants and products at equal rates meaning there is no net change
Reversible reactions that happen in closed systems eventually reach a dynamic equilibrium At this point the forward and reverse reactions still carry on dynamic but they happen at an equal rate and so the concentrations of all
Printables for free have gained immense recognition for a variety of compelling motives:
-
Cost-Effective: They eliminate the need to buy physical copies or costly software.
-
Modifications: There is the possibility of tailoring designs to suit your personal needs be it designing invitations to organize your schedule or even decorating your house.
-
Educational Value: Educational printables that can be downloaded for free cater to learners of all ages, which makes them an invaluable tool for parents and educators.
-
It's easy: instant access various designs and templates helps save time and effort.
Where to Find more What Is Dynamic Equilibrium Reactions
13 1 Chemical Equilibria Chemistry 112 Chapters 12 17 Of OpenStax
13 1 Chemical Equilibria Chemistry 112 Chapters 12 17 Of OpenStax
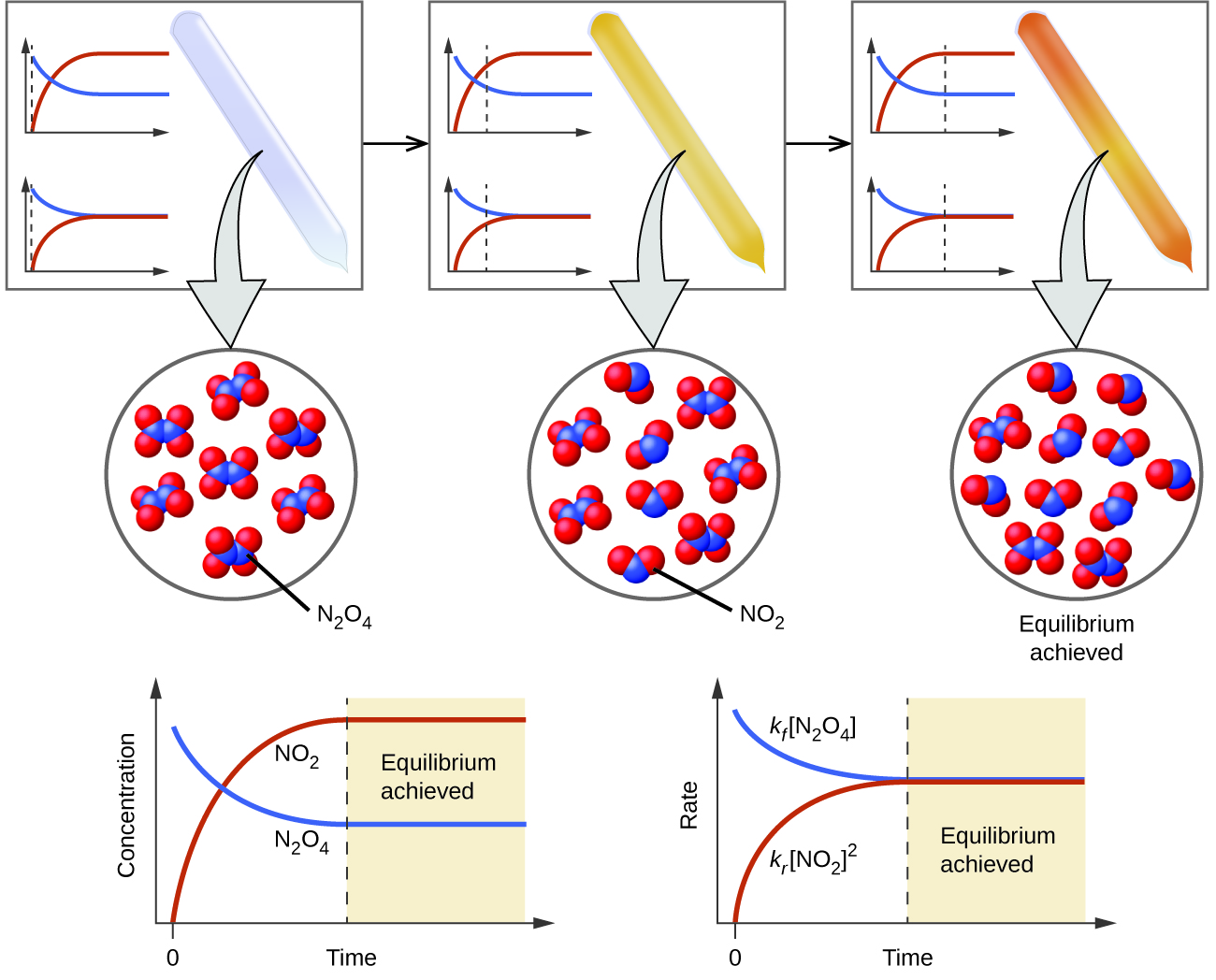
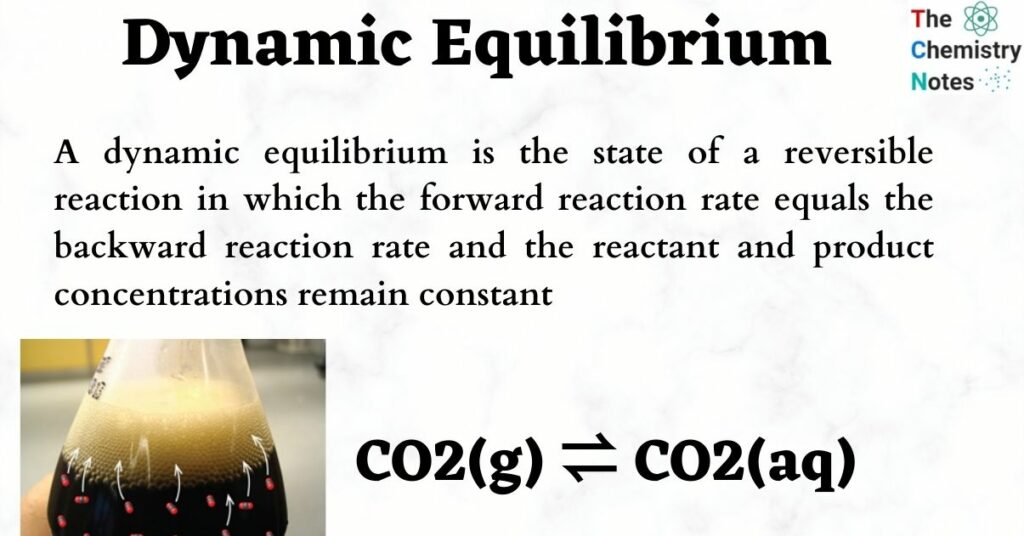
Dynamic equilibrium is the point at which a chemical reaction continues to proceed both forward and in reverse but there is no net change in the amount of reactants and products
A dynamic equilibrium is a chemical equilibrium between a forward reaction and the reverse reaction where the rate of the reactions are equal At this point the ratio between reactants and products remains
If we've already piqued your curiosity about What Is Dynamic Equilibrium Reactions we'll explore the places you can locate these hidden treasures:
1. Online Repositories
- Websites like Pinterest, Canva, and Etsy provide a variety of What Is Dynamic Equilibrium Reactions to suit a variety of purposes.
- Explore categories like home decor, education, the arts, and more.
2. Educational Platforms
- Educational websites and forums often offer worksheets with printables that are free including flashcards, learning tools.
- The perfect resource for parents, teachers and students in need of additional resources.
3. Creative Blogs
- Many bloggers share their creative designs and templates for no cost.
- The blogs covered cover a wide selection of subjects, including DIY projects to party planning.
Maximizing What Is Dynamic Equilibrium Reactions
Here are some fresh ways of making the most of printables for free:
1. Home Decor
- Print and frame gorgeous art, quotes, as well as seasonal decorations, to embellish your living areas.
2. Education
- Utilize free printable worksheets to enhance your learning at home or in the classroom.
3. Event Planning
- Create invitations, banners, and decorations for special events such as weddings and birthdays.
4. Organization
- Keep your calendars organized by printing printable calendars along with lists of tasks, and meal planners.
Conclusion
What Is Dynamic Equilibrium Reactions are a treasure trove of useful and creative resources that meet a variety of needs and needs and. Their availability and versatility make them a fantastic addition to both personal and professional life. Explore the vast array of What Is Dynamic Equilibrium Reactions and unlock new possibilities!
Frequently Asked Questions (FAQs)
-
Are What Is Dynamic Equilibrium Reactions truly for free?
- Yes, they are! You can download and print the resources for free.
-
Can I use free printing templates for commercial purposes?
- It's all dependent on the conditions of use. Make sure you read the guidelines for the creator before using printables for commercial projects.
-
Do you have any copyright concerns with What Is Dynamic Equilibrium Reactions?
- Some printables could have limitations regarding usage. Be sure to read the terms of service and conditions provided by the designer.
-
How do I print printables for free?
- You can print them at home using printing equipment or visit a local print shop to purchase premium prints.
-
What software do I need in order to open printables free of charge?
- The majority of printables are in the format PDF. This can be opened with free software, such as Adobe Reader.
Dynamic Equilibrium Definition Important Examples
Chemical Equilibrum Passnownow

Check more sample of What Is Dynamic Equilibrium Reactions below
7 Important Difference Between Static And Dynamic Equilibrium With

Dynamics Equilibrium With Examples Physics Tutorials

Chemical Equilibrium I Types Of Equilibrium Equilibrium Constant

W02M04 Dynamic Equilibrium Equation By Energy Method YouTube

Difference Between Static And Dynamic Equilibrium Equilibrium

Chemical Equilibrium 838 Plays Quizizz

https://www.bbc.co.uk/bitesize/guides/zwv…
Dynamic equilibrium Many chemical reactions are reversible In these reactions there is both a forward reaction where reactants are made into products and a reverse reaction where
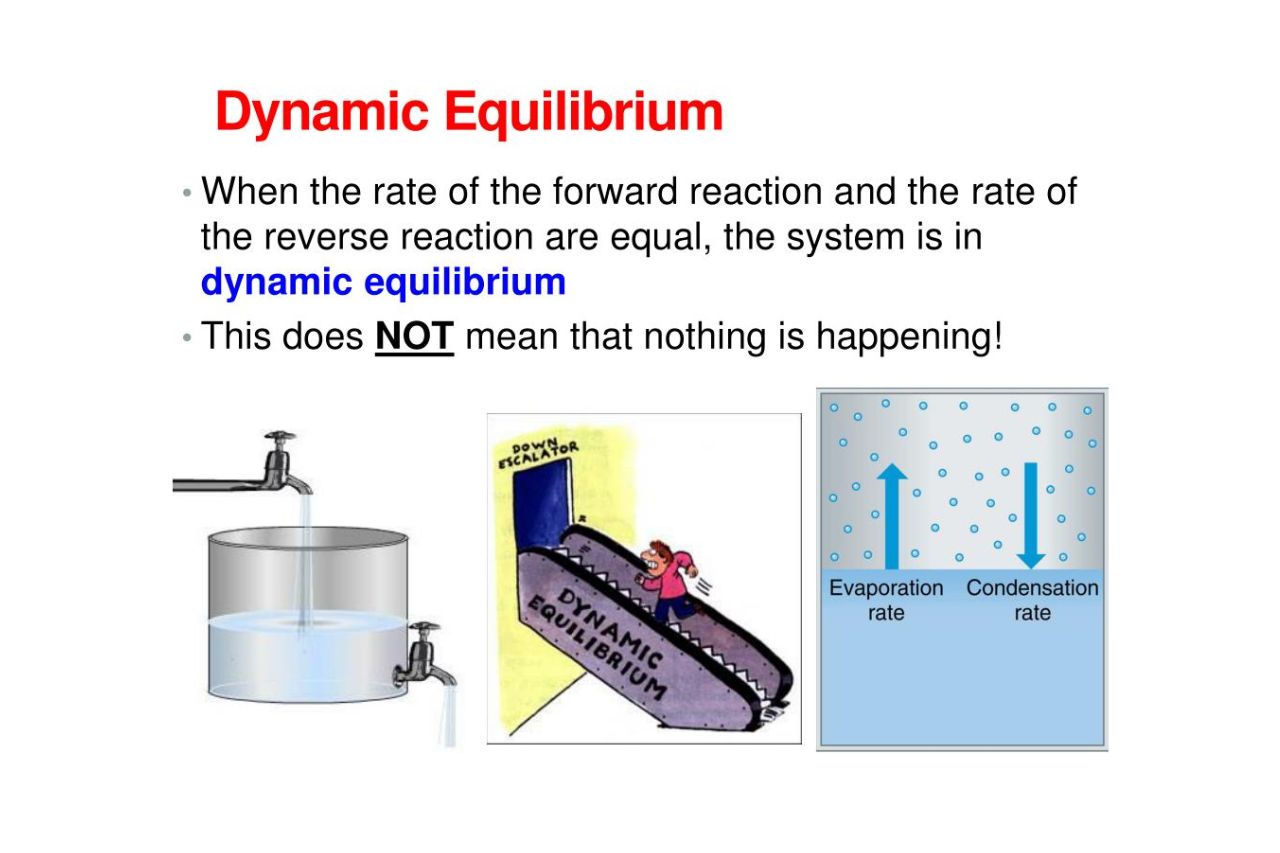
https://chem.libretexts.org/Bookshelves/…
Chemical equilibrium is a dynamic process that consists of a forward reaction in which reactants are converted to products and a reverse reaction in which products are converted to reactants At equilibrium the
Dynamic equilibrium Many chemical reactions are reversible In these reactions there is both a forward reaction where reactants are made into products and a reverse reaction where
Chemical equilibrium is a dynamic process that consists of a forward reaction in which reactants are converted to products and a reverse reaction in which products are converted to reactants At equilibrium the

W02M04 Dynamic Equilibrium Equation By Energy Method YouTube

Dynamics Equilibrium With Examples Physics Tutorials

Difference Between Static And Dynamic Equilibrium Equilibrium
Chemical Equilibrium 838 Plays Quizizz
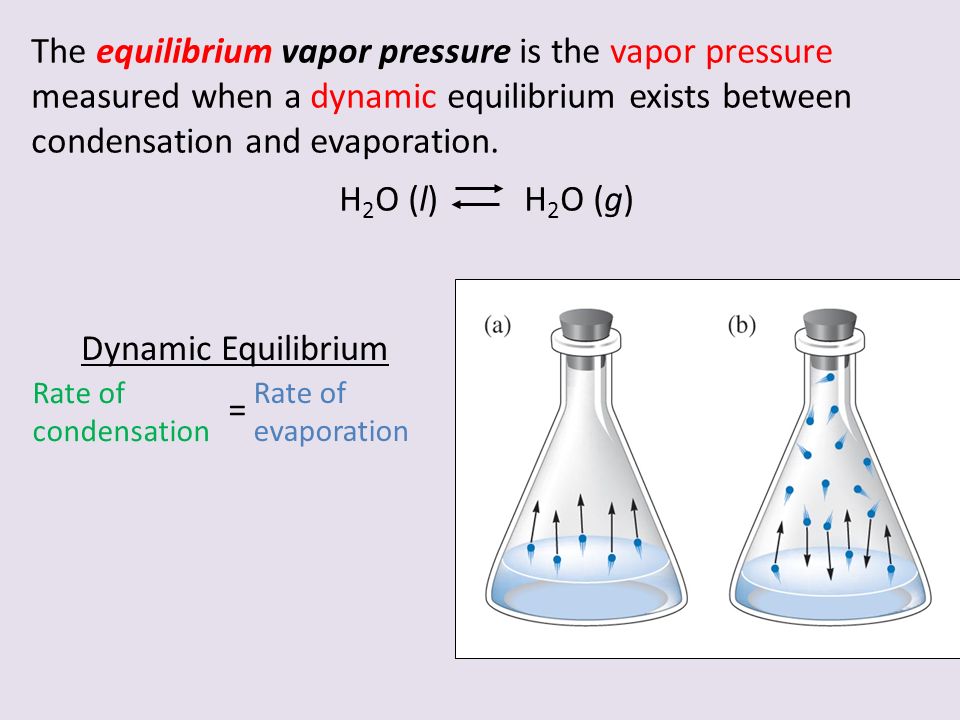
What Do We Mean By A Dynamic Equilibrium Can You Describe How The

Dynamic Chemical Equilibrium Definition Examples Video Lesson

Dynamic Chemical Equilibrium Definition Examples Video Lesson
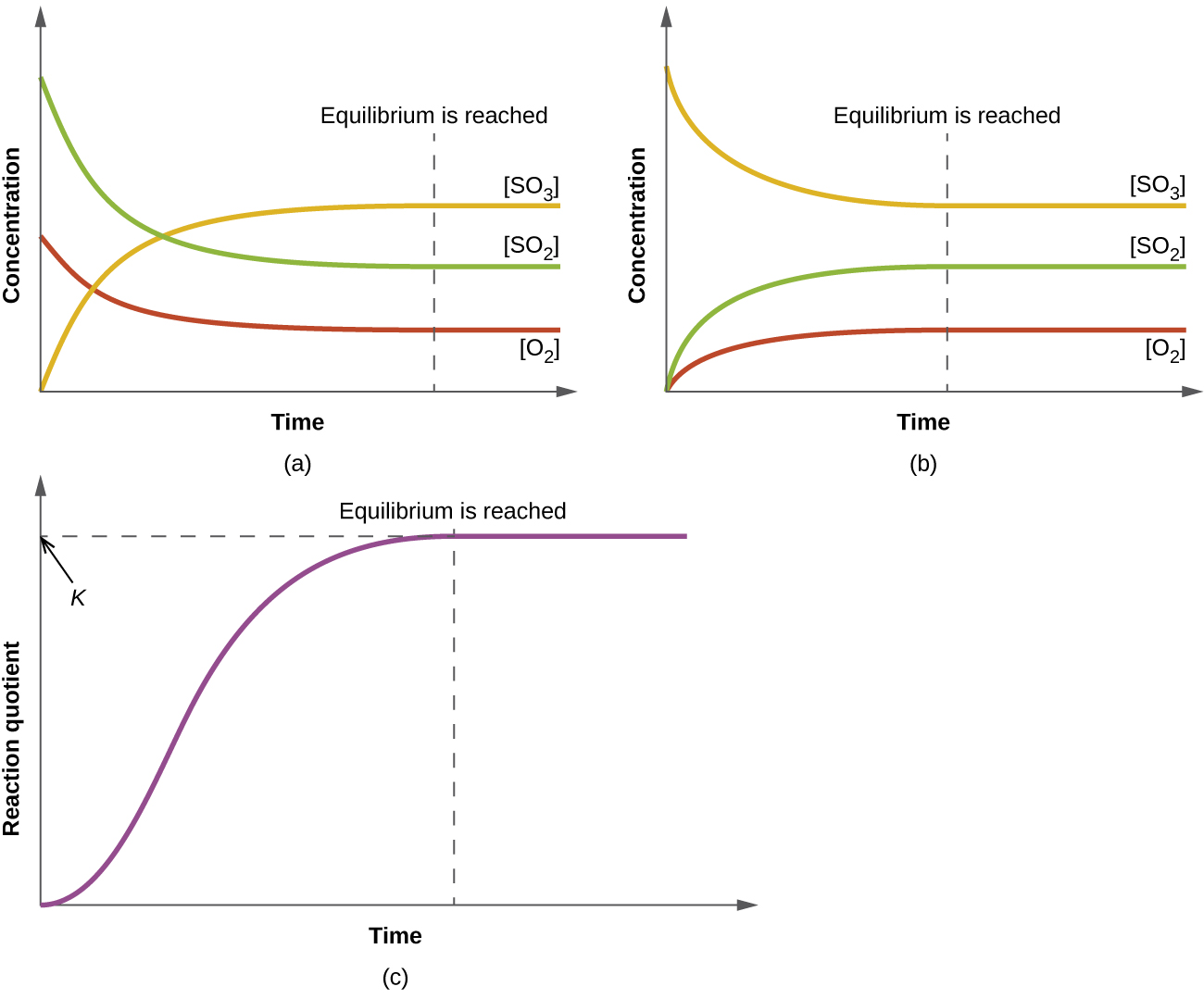
13 2 Equilibrium Constants Chemistry 112 Chapters 12 17 Of OpenStax