In this day and age where screens rule our lives however, the attraction of tangible printed materials hasn't faded away. If it's to aid in education and creative work, or just adding an extra personal touch to your home, printables for free have become an invaluable source. In this article, we'll take a dive to the depths of "What Is Dynamic Equilibrium In Chemistry," exploring the different types of printables, where they are available, and how they can be used to enhance different aspects of your life.
Get Latest What Is Dynamic Equilibrium In Chemistry Below

What Is Dynamic Equilibrium In Chemistry
What Is Dynamic Equilibrium In Chemistry - What Is Dynamic Equilibrium In Chemistry, What Is Dynamic Equilibrium In Chemistry Class 10, What Is Dynamic Equilibrium In Chemistry Gcse, What Is Dynamic Equilibrium In Chemistry Class 12, What Is Meant By Dynamic Equilibrium In Chemistry, What Is Dynamic Equilibrium Reactions Chemistry Fuseschool, What Is Dynamic Equilibrium A Level Chemistry, What Does Dynamic Equilibrium Mean In Chemistry, What Is Dynamic Equilibrium Reversible Reaction, What Is The Difference Between Static And Dynamic Equilibrium In Chemistry
Dynamic equilibrium Many chemical reactions are reversible In these reactions there is both a forward reaction where reactants are made into products and a reverse reaction where
Dynamic equilibrium occurs when for a reversible reaction the rate of the forward reaction equals the rate of the reverse reaction Since the two rates are equal it looks like nothing is happening but in reality the reaction is
What Is Dynamic Equilibrium In Chemistry offer a wide assortment of printable materials online, at no cost. They are available in numerous types, such as worksheets coloring pages, templates and more. The beauty of What Is Dynamic Equilibrium In Chemistry is in their variety and accessibility.
More of What Is Dynamic Equilibrium In Chemistry
PPT Dynamic Equilibrium PowerPoint Presentation Free Download ID
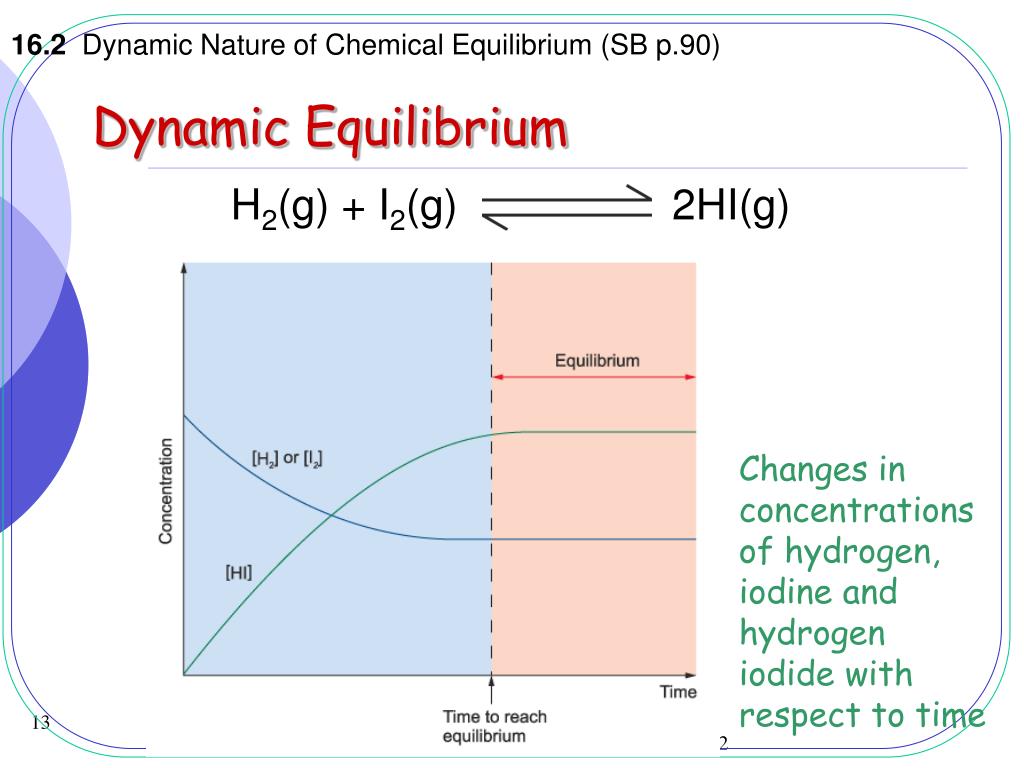
PPT Dynamic Equilibrium PowerPoint Presentation Free Download ID
Chemical equilibrium occurs when the number of particles becoming products is equal to the number of particles becoming reactants A dynamic equilibrium is a state where the rate of the forward reaction is equal to the rate of the reverse reaction
A dynamic equilibrium is a chemical equilibrium between a forward reaction and the reverse reaction where the rate of the reactions are equal At this point the ratio between reactants and products remains unchanged over time
Print-friendly freebies have gained tremendous appeal due to many compelling reasons:
-
Cost-Effective: They eliminate the requirement to purchase physical copies or costly software.
-
Flexible: Your HTML0 customization options allow you to customize printed materials to meet your requirements for invitations, whether that's creating them as well as organizing your calendar, or even decorating your home.
-
Educational value: Downloads of educational content for free cater to learners of all ages. This makes them an essential instrument for parents and teachers.
-
Convenience: immediate access a plethora of designs and templates saves time and effort.
Where to Find more What Is Dynamic Equilibrium In Chemistry
Chemical Equilibrum Passnownow
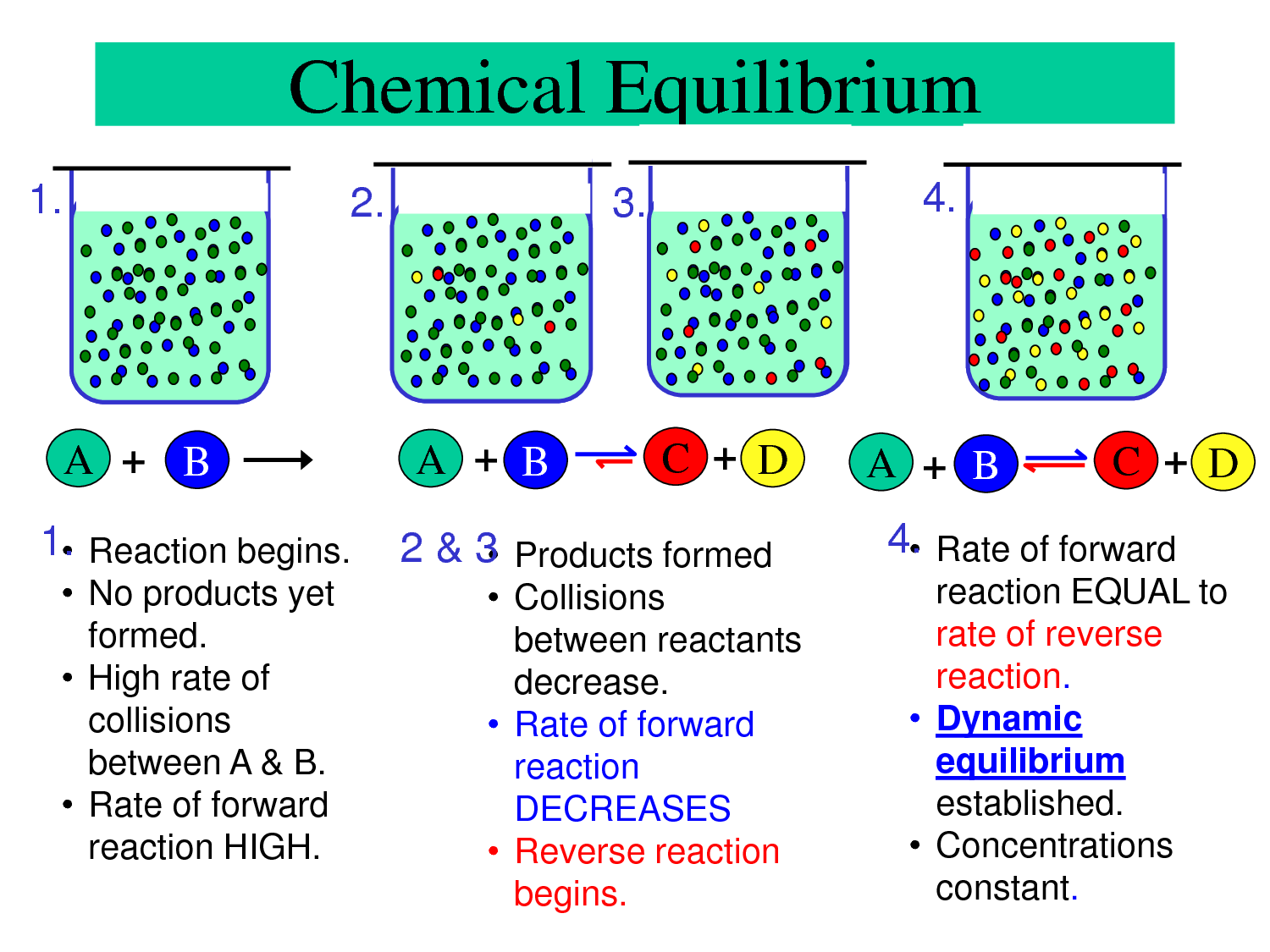
Chemical Equilibrum Passnownow
Key Questions How does concentration effect dynamic equilibrium Answer The Law of Mass Action states that when a system e g chemical reaction is at equilibrium the ratio of products and reactant concentrations is equal to the equilibrium constant for that reaction
A reaction is at equilibrium when the amounts of reactants or products no longer change Chemical equilibrium is a dynamic process meaning the rate of formation of products by the forward reaction is equal to the rate at which the products re form reactants by the reverse reaction
We hope we've stimulated your curiosity about What Is Dynamic Equilibrium In Chemistry Let's look into where you can find these elusive treasures:
1. Online Repositories
- Websites like Pinterest, Canva, and Etsy offer a vast selection of What Is Dynamic Equilibrium In Chemistry to suit a variety of uses.
- Explore categories such as home decor, education, management, and craft.
2. Educational Platforms
- Forums and educational websites often provide free printable worksheets along with flashcards, as well as other learning materials.
- Perfect for teachers, parents as well as students who require additional resources.
3. Creative Blogs
- Many bloggers offer their unique designs and templates, which are free.
- The blogs are a vast variety of topics, that range from DIY projects to planning a party.
Maximizing What Is Dynamic Equilibrium In Chemistry
Here are some inventive ways that you can make use of What Is Dynamic Equilibrium In Chemistry:
1. Home Decor
- Print and frame beautiful art, quotes, or even seasonal decorations to decorate your living areas.
2. Education
- Use free printable worksheets to help reinforce your learning at home or in the classroom.
3. Event Planning
- Create invitations, banners, and decorations for special occasions such as weddings, birthdays, and other special occasions.
4. Organization
- Keep your calendars organized by printing printable calendars checklists for tasks, as well as meal planners.
Conclusion
What Is Dynamic Equilibrium In Chemistry are a treasure trove filled with creative and practical information that cater to various needs and passions. Their availability and versatility make them an invaluable addition to the professional and personal lives of both. Explore the vast collection that is What Is Dynamic Equilibrium In Chemistry today, and uncover new possibilities!
Frequently Asked Questions (FAQs)
-
Are What Is Dynamic Equilibrium In Chemistry really gratis?
- Yes, they are! You can download and print these items for free.
-
Can I make use of free printing templates for commercial purposes?
- It's based on the terms of use. Always review the terms of use for the creator prior to printing printables for commercial projects.
-
Are there any copyright concerns when using printables that are free?
- Certain printables might have limitations in use. Make sure you read the terms of service and conditions provided by the designer.
-
How do I print printables for free?
- You can print them at home using either a printer at home or in a print shop in your area for premium prints.
-
What software is required to open printables that are free?
- Many printables are offered in the format of PDF, which can be opened with free software, such as Adobe Reader.
Equilibrium Dynamic Equilibrium Wyzant Resources

PPT Chemical Equilibrium PowerPoint Presentation ID 3889861
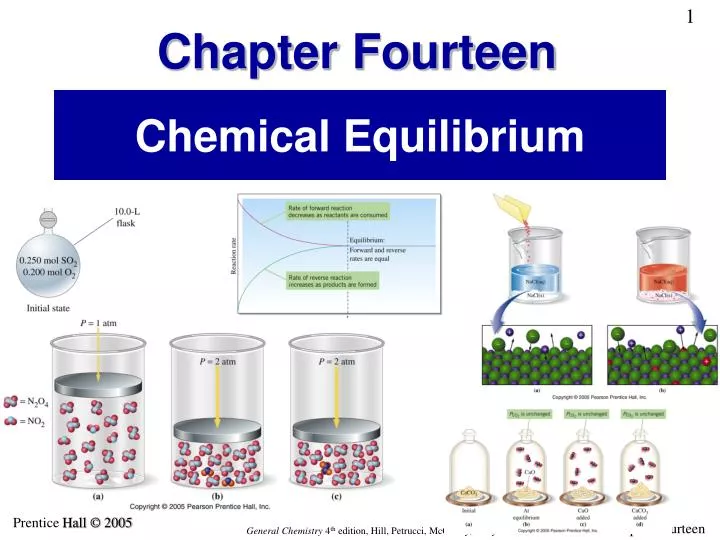
Check more sample of What Is Dynamic Equilibrium In Chemistry below
Difference Between Static And Dynamic Equilibrium Equilibrium

Dynamic Equilibrium YouTube
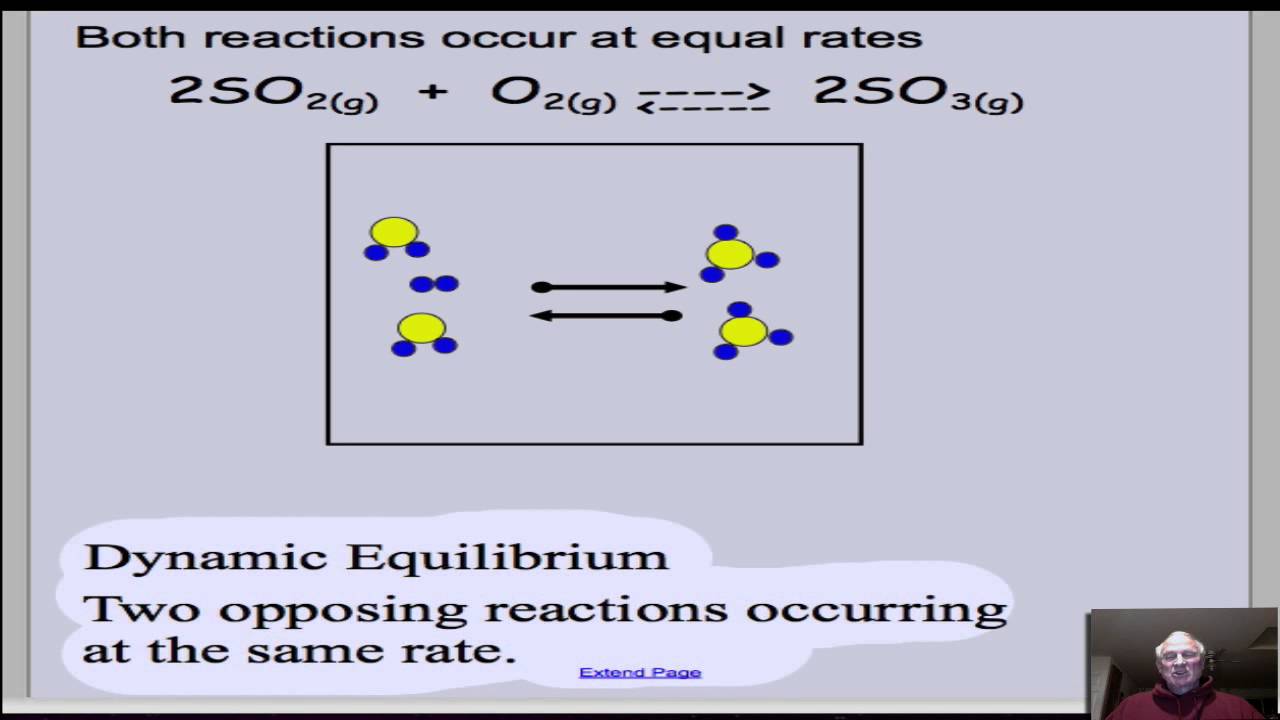
13 1 Chemical Equilibria Chemistry 112 Chapters 12 17 Of OpenStax
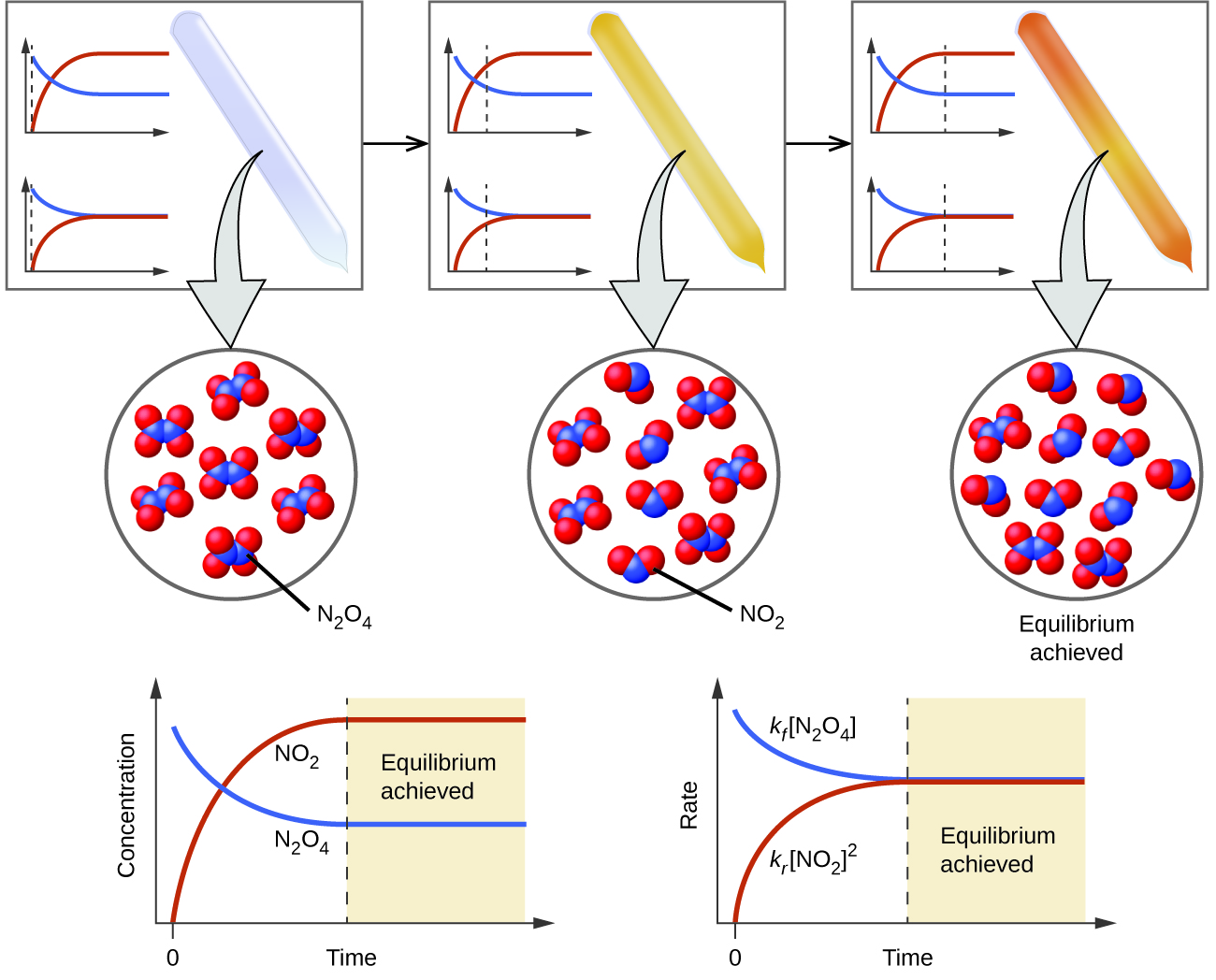
Chemical Equilibrium Chemistry Steps
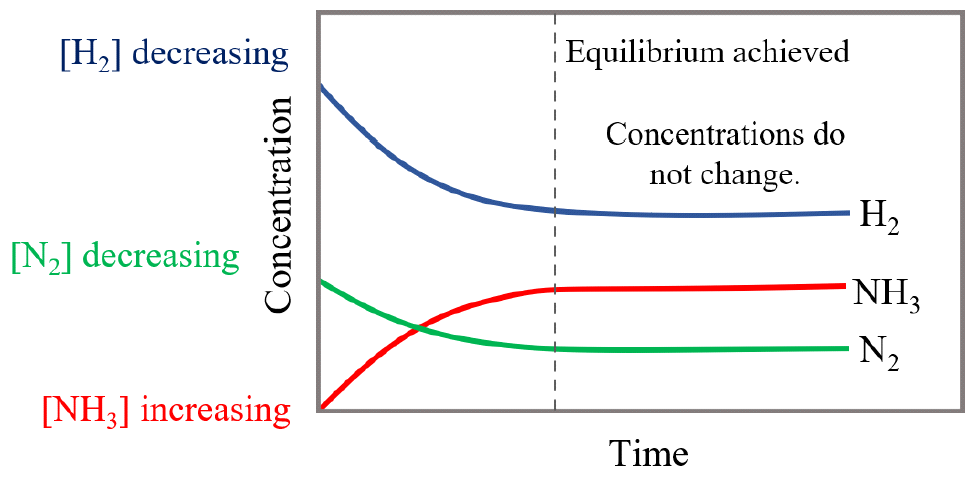
7 Important Difference Between Static And Dynamic Equilibrium With

Chemical Equilibrium 809 Plays Quizizz

https://blog.prepscholar.com/what-is-dynamic...
Dynamic equilibrium occurs when for a reversible reaction the rate of the forward reaction equals the rate of the reverse reaction Since the two rates are equal it looks like nothing is happening but in reality the reaction is

https://chem.libretexts.org/Bookshelves/General...
Chemical equilibrium is a dynamic process that consists of a forward reaction in which reactants are converted to products and a reverse reaction in which products are converted to reactants At equilibrium the forward and reverse reactions proceed at equal rates
Dynamic equilibrium occurs when for a reversible reaction the rate of the forward reaction equals the rate of the reverse reaction Since the two rates are equal it looks like nothing is happening but in reality the reaction is
Chemical equilibrium is a dynamic process that consists of a forward reaction in which reactants are converted to products and a reverse reaction in which products are converted to reactants At equilibrium the forward and reverse reactions proceed at equal rates

Chemical Equilibrium Chemistry Steps

Dynamic Equilibrium YouTube

7 Important Difference Between Static And Dynamic Equilibrium With
Chemical Equilibrium 809 Plays Quizizz

Chemical Equilibrium I Types Of Equilibrium Equilibrium Constant
Chemical Equilibrium What Are The Characteristics Of Equilibrium

Chemical Equilibrium What Are The Characteristics Of Equilibrium

Dynamic Chemical Equilibrium Definition Examples Video Lesson