In this age of technology, in which screens are the norm yet the appeal of tangible printed objects hasn't waned. If it's to aid in education in creative or artistic projects, or simply adding some personal flair to your area, Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers are now a useful resource. In this article, we'll take a dive into the world of "Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers," exploring the benefits of them, where to find them and how they can enrich various aspects of your lives.
Get Latest Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers Below

Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers
Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers - Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers
A determine the limiting reagent b determine the number of moles of H 2O produced c determine the number of grams of CaSO 4 produced d determine the number of grams of excess reagent left 1 make sure the equation is balanced This equation is already balanced 2 then determine the moles of each compound that you have
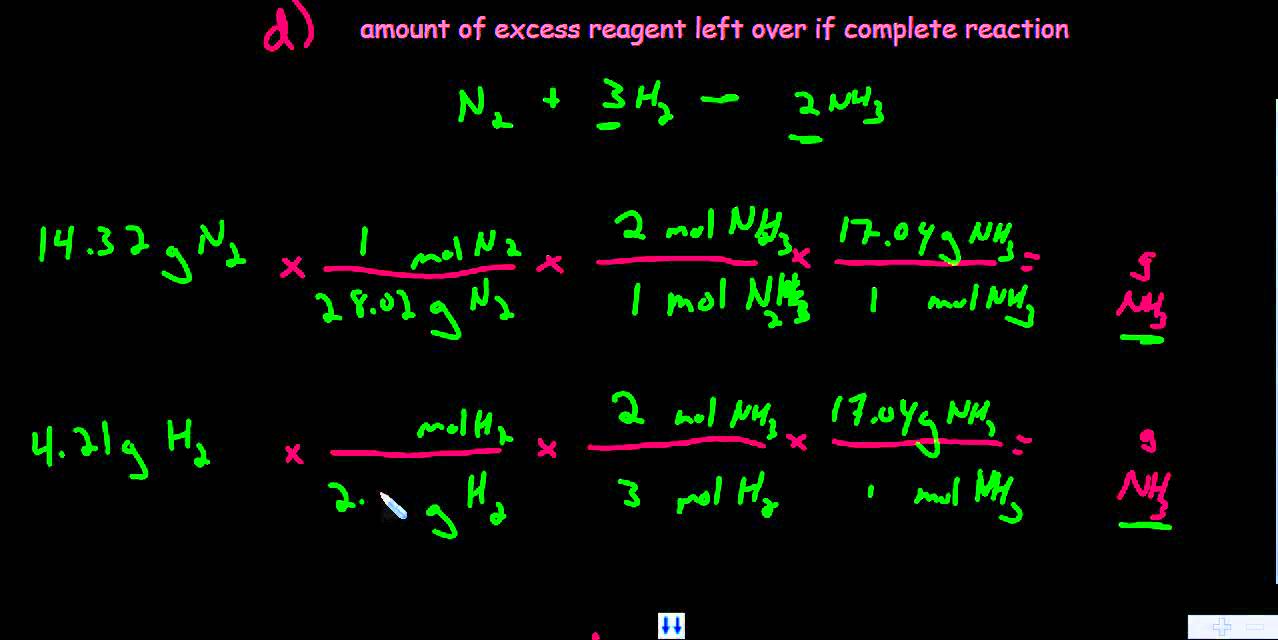
LIMITING REAGENTS THEORETICAL ACTUAL AND PERCENT YIELDS EXAMPLE OF A LIMITING REAGENT PROBLEM How many grams of NH 3 can be be produced theoretically from the reaction of 5 0 g of N What is the limiting reagent If 8 52 g are actually formed what is the percent yield of NH 3 Balance 3 H 2 N 2 o 2 NH 2 and
The Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers are a huge range of printable, free material that is available online at no cost. These materials come in a variety of designs, including worksheets coloring pages, templates and many more. The beauty of Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers is in their versatility and accessibility.
More of Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers
Limiting Reactant Excess Reactant Theoretical Yield And Percent Yield
Limiting Reactant Excess Reactant Theoretical Yield And Percent Yield
1 LiOH KCl LiCl KOH a I began this reaction with 20 grams of lithium hydroxide What is my theoretical yield of lithium chloride 35 5 grams b I actually produced 6 grams of lithium chloride What is my percent yield 16 9 2 C3H8 5 O2 3 CO2 4 H2O a If I start with 5 grams of C3H8 what is my theoretical yield of water
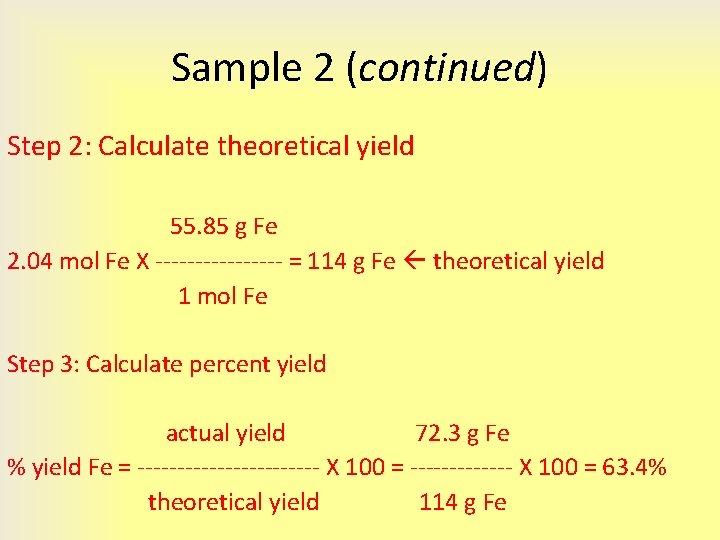
Percent yield actual yield theoretical yield 100 Based on this definition we would expect a percent yield to have a value between 0 and 100 If our percent yield is greater than 100 that means we probably calculated something incorrectly or made an experimental error
Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers have gained a lot of appeal due to many compelling reasons:
-
Cost-Efficiency: They eliminate the requirement to purchase physical copies or costly software.
-
Personalization They can make printables to your specific needs whether it's making invitations or arranging your schedule or decorating your home.
-
Educational Worth: Educational printables that can be downloaded for free provide for students of all ages, making them an invaluable tool for parents and educators.
-
Affordability: Fast access a myriad of designs as well as templates is time-saving and saves effort.
Where to Find more Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers
Limiting Reactant And Percent Yield Practice Worksheet Answer Key

Limiting Reactant And Percent Yield Practice Worksheet Answer Key
Determine the theoretical yield of Al2O3 160 0 g Al x 1 mol Al x 2 mol Al2O3 x 101 964 g Al2O3 302 3 g Al2O3 26 982 g Al 4 mol Al 1 mol Al2O3 c Determine the percent yield yield actual x 100 260 0 g x 100 86 01 Theoretical 302 3 g
Limiting Reagent Percent Yield Practice Worksheet 1 When copper II chloride reacts with sodium nitrate copper II nitrate and sodium chloride are formed a Write the balanced equation for the reaction given above CuCl2 NaNO3 Cu NO3 2 NaCl CuCl2 2 NaNO3 Cu NO3 2 2 NaCl b
Now that we've piqued your interest in printables for free Let's see where you can discover these hidden gems:
1. Online Repositories
- Websites such as Pinterest, Canva, and Etsy offer a huge selection with Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers for all purposes.
- Explore categories such as the home, decor, organisation, as well as crafts.
2. Educational Platforms
- Educational websites and forums frequently provide worksheets that can be printed for free including flashcards, learning materials.
- It is ideal for teachers, parents and students looking for extra sources.
3. Creative Blogs
- Many bloggers are willing to share their original designs as well as templates for free.
- The blogs are a vast spectrum of interests, starting from DIY projects to party planning.
Maximizing Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers
Here are some ways for you to get the best of printables for free:
1. Home Decor
- Print and frame gorgeous artwork, quotes, and seasonal decorations, to add a touch of elegance to your living spaces.
2. Education
- Use printable worksheets for free for reinforcement of learning at home (or in the learning environment).
3. Event Planning
- Create invitations, banners, and other decorations for special occasions like weddings or birthdays.
4. Organization
- Stay organized with printable calendars along with lists of tasks, and meal planners.
Conclusion
Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers are a treasure trove filled with creative and practical information designed to meet a range of needs and desires. Their access and versatility makes them a valuable addition to each day life. Explore the plethora of Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers right now and discover new possibilities!
Frequently Asked Questions (FAQs)
-
Are printables for free really gratis?
- Yes they are! You can download and print these resources at no cost.
-
Do I have the right to use free printables for commercial uses?
- It's contingent upon the specific rules of usage. Be sure to read the rules of the creator before utilizing printables for commercial projects.
-
Do you have any copyright issues in printables that are free?
- Certain printables could be restricted on use. Always read these terms and conditions as set out by the designer.
-
How can I print Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers?
- Print them at home with any printer or head to a local print shop to purchase the highest quality prints.
-
What software do I need to run printables that are free?
- The majority of PDF documents are provided in the PDF format, and is open with no cost programs like Adobe Reader.
Reactierendement Berekenen 13 Stappen met Afbeeldingen WikiHow

Theoretical Yield BREAKING NEWS Reagents Must Be Limited They re

Check more sample of Limiting Reagents Theoretical Actual And Percent Yield Worksheet Answers below
Percent Yield WS

Stoichiometry Percent Yield Worksheet Show All Work Answers Printable

4 3 Limiting Reactant Theoretical Yield And Percent
Limiting Reagent And Percent Yield Worksheet

Limiting Reactant And Percent Yield Worksheet s

Theoretical And Percent Yield Worksheet Answers

https://www.csun.edu/~hcchm001/LIMITREG.pdf
LIMITING REAGENTS THEORETICAL ACTUAL AND PERCENT YIELDS EXAMPLE OF A LIMITING REAGENT PROBLEM How many grams of NH 3 can be be produced theoretically from the reaction of 5 0 g of N What is the limiting reagent If 8 52 g are actually formed what is the percent yield of NH 3 Balance 3 H 2 N 2 o 2 NH 2 and

https://chem.libretexts.org/Bookshelves/General...
The percent yield of a reaction is the ratio of the actual yield to the theoretical yield multiplied by 100 to give a percentage percent yield actual yield g theoretical yield g 100 The method used to calculate the percent yield of a reaction is illustrated in Example 4 3 4
LIMITING REAGENTS THEORETICAL ACTUAL AND PERCENT YIELDS EXAMPLE OF A LIMITING REAGENT PROBLEM How many grams of NH 3 can be be produced theoretically from the reaction of 5 0 g of N What is the limiting reagent If 8 52 g are actually formed what is the percent yield of NH 3 Balance 3 H 2 N 2 o 2 NH 2 and
The percent yield of a reaction is the ratio of the actual yield to the theoretical yield multiplied by 100 to give a percentage percent yield actual yield g theoretical yield g 100 The method used to calculate the percent yield of a reaction is illustrated in Example 4 3 4

Limiting Reagent And Percent Yield Worksheet

Stoichiometry Percent Yield Worksheet Show All Work Answers Printable

Limiting Reactant And Percent Yield Worksheet s

Theoretical And Percent Yield Worksheet Answers

Limiting Reactant Worksheet Answers Beautiful Limiting Reagent

Limiting Reagent Theoretical And Actual Yields Worksheet

Limiting Reagent Theoretical And Actual Yields Worksheet

Limiting Reactant And Percent Yield Worksheet s