In this day and age with screens dominating our lives but the value of tangible printed items hasn't gone away. It doesn't matter if it's for educational reasons as well as creative projects or simply adding an individual touch to your space, Ion Electron Method can be an excellent source. Here, we'll dive into the world "Ion Electron Method," exploring what they are, how to get them, as well as how they can add value to various aspects of your lives.
Get Latest Ion Electron Method Below
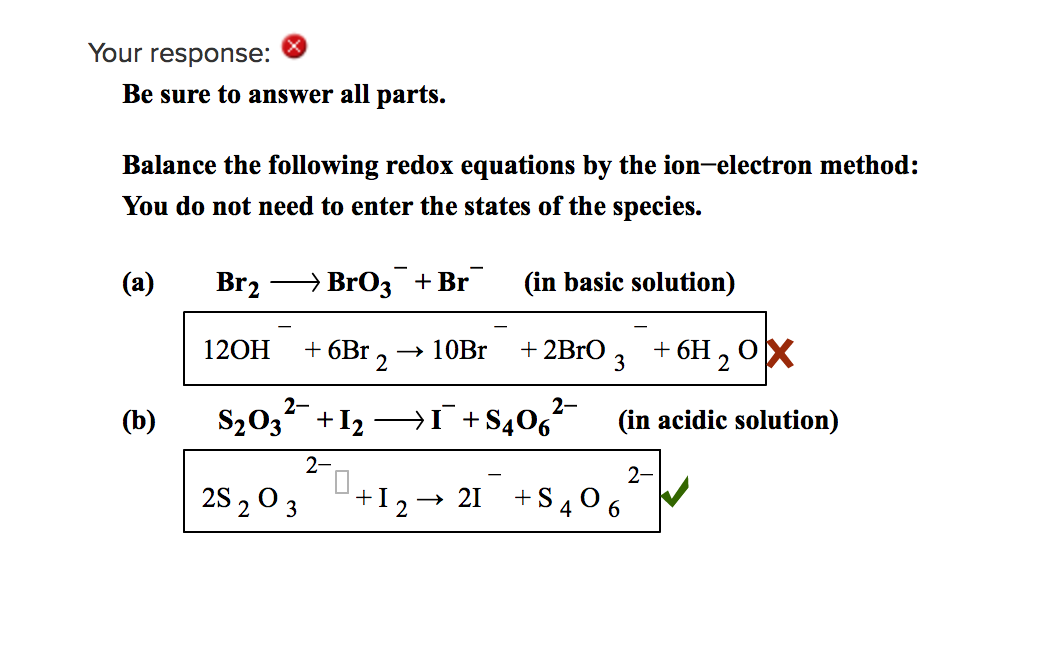
Ion Electron Method
Ion Electron Method - Ion Electron Method, Ion Electron Method Calculator, Ion Electron Method Class 11 Questions, Ion Electron Method In Basic Medium, Ion Electron Method In Acidic Medium, Ion Electron Method Is Also Known As, Ion Electron Method Steps, Ion Electron Method For Balancing Redox Reaction, Ion Electron Method Example, Ion Electron Method In Hindi
The oxidation number is the number of valence electrons an atom is assumed to have when the electrons are counted according to certain arbitrary rules Two important rules are 1 The oxidation number of O in its compounds is almost always 2 2 The charge on a molecule or ion is equal to the sum of the oxidation numbers of its atoms
Use the ion electron method to complete and balance the following skeletal redox equations occurring in either acidic or basic aqueous solution as indicated Identify the oxidation and reduction half reactions in each case In acidic aqueous solution Cu NO3 rightarrow Cu 2 N 2O 4
Ion Electron Method offer a wide assortment of printable content that can be downloaded from the internet at no cost. They come in many types, such as worksheets templates, coloring pages, and much more. The value of Ion Electron Method lies in their versatility as well as accessibility.
More of Ion Electron Method
PPT Chapter 6 Oxidation Reduction Reactions PowerPoint Presentation ID 4012495

PPT Chapter 6 Oxidation Reduction Reactions PowerPoint Presentation ID 4012495
The ion electron method allows one to balance redox reactions regardless of their complexity We illustrate this method with two examples Example 1 I is oxidized to IO 3 by MnO 4 which is reduced to Mn 2 How can this reaction be balanced In the ion electron method we follow a series of four steps
The first step to balance any redox reaction is to separate the reaction into half reactions The substance being reduced will have electrons as reactants and the oxidized substance will have electrons as products Usually all reactions are written as reduction reactions in half reaction tables
Ion Electron Method have gained a lot of recognition for a variety of compelling motives:
-
Cost-Effective: They eliminate the necessity of purchasing physical copies of the software or expensive hardware.
-
Personalization This allows you to modify printables to fit your particular needs such as designing invitations or arranging your schedule or even decorating your home.
-
Educational Benefits: These Ion Electron Method offer a wide range of educational content for learners of all ages. This makes them a useful tool for parents and teachers.
-
Convenience: Access to an array of designs and templates helps save time and effort.
Where to Find more Ion Electron Method
RR 4 I Redox Reactions I Balancing Redox Reactions Using Ion Electron Method I

RR 4 I Redox Reactions I Balancing Redox Reactions Using Ion Electron Method I
Live Classes Video Lectures Test Series Lecturewise notes topicwise DPP dynamic Exercise and much more on Physicswallah App Download the App from Google
The Half Reaction Method First separate the equation into two half reactions the oxidation portion and the reduction portion This is called the half reaction method of balancing redox reactions or the ion electron method Each half reaction is balanced separately and then the equations are added together to give a balanced
Now that we've piqued your interest in Ion Electron Method Let's see where you can discover these hidden gems:
1. Online Repositories
- Websites such as Pinterest, Canva, and Etsy provide a variety of printables that are free for a variety of goals.
- Explore categories like decorating your home, education, crafting, and organization.
2. Educational Platforms
- Forums and educational websites often provide worksheets that can be printed for free for flashcards, lessons, and worksheets. materials.
- This is a great resource for parents, teachers or students in search of additional sources.
3. Creative Blogs
- Many bloggers offer their unique designs and templates for free.
- These blogs cover a broad variety of topics, all the way from DIY projects to planning a party.
Maximizing Ion Electron Method
Here are some innovative ways for you to get the best of Ion Electron Method:
1. Home Decor
- Print and frame gorgeous artwork, quotes, or seasonal decorations to adorn your living areas.
2. Education
- Print out free worksheets and activities for reinforcement of learning at home for the classroom.
3. Event Planning
- Design invitations, banners and other decorations for special occasions such as weddings and birthdays.
4. Organization
- Get organized with printable calendars along with lists of tasks, and meal planners.
Conclusion
Ion Electron Method are a treasure trove of creative and practical resources designed to meet a range of needs and preferences. Their access and versatility makes they a beneficial addition to both professional and personal lives. Explore the vast world of Ion Electron Method now and unlock new possibilities!
Frequently Asked Questions (FAQs)
-
Are Ion Electron Method really free?
- Yes you can! You can download and print these tools for free.
-
Do I have the right to use free printouts for commercial usage?
- It is contingent on the specific rules of usage. Be sure to read the rules of the creator before utilizing printables for commercial projects.
-
Do you have any copyright problems with Ion Electron Method?
- Some printables may have restrictions regarding their use. Make sure you read the terms and regulations provided by the creator.
-
How do I print Ion Electron Method?
- Print them at home with a printer or visit an in-store print shop to get the highest quality prints.
-
What program must I use to open printables free of charge?
- The majority of PDF documents are provided in PDF format. They is open with no cost software, such as Adobe Reader.
Chemistry Archive July 10 2015 Chegg
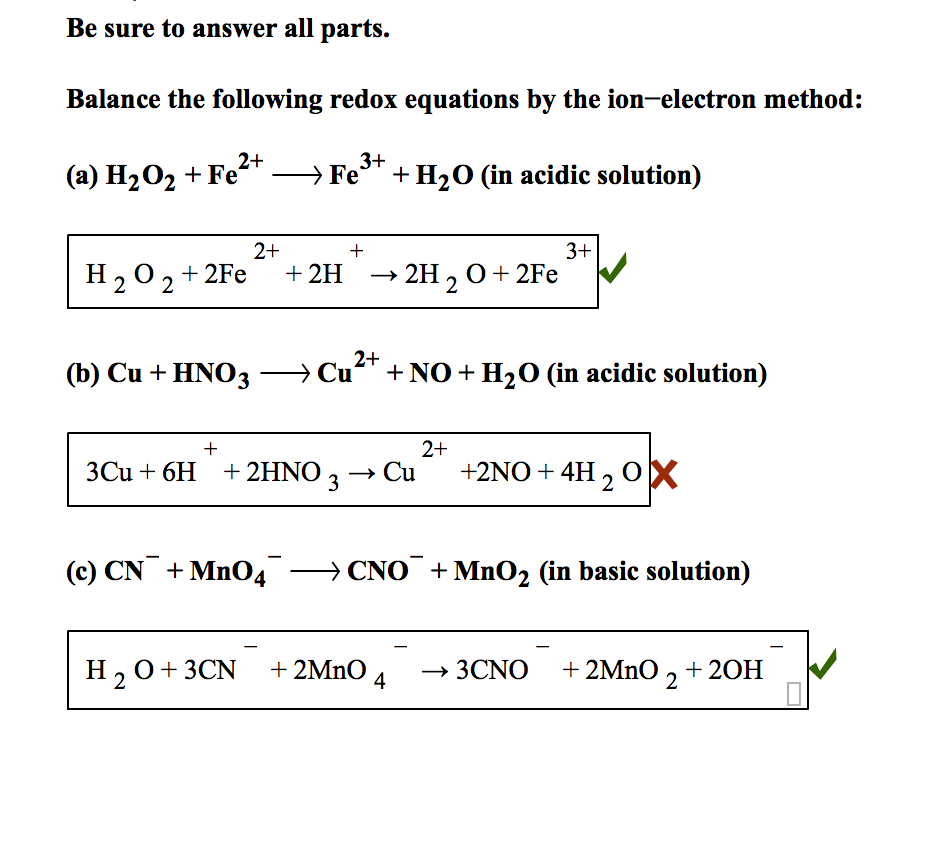
Ion Electron Method YouTube
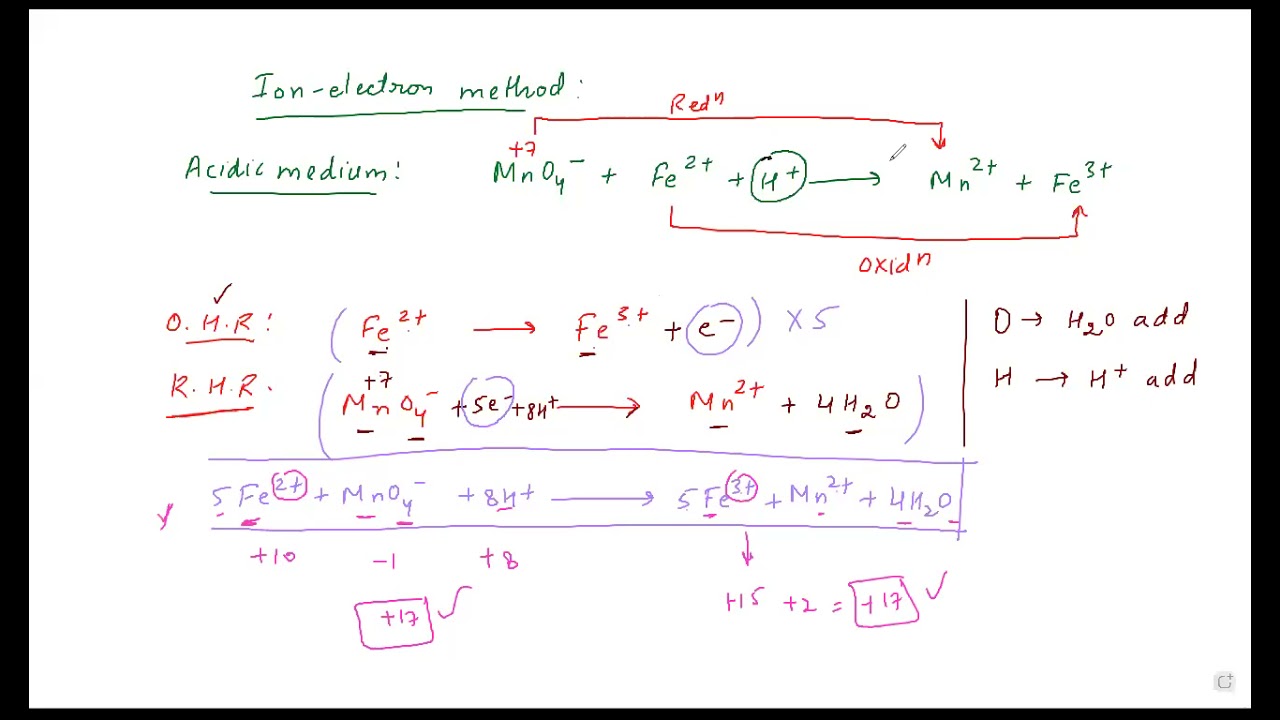
Check more sample of Ion Electron Method below
Ion Electron Method For Balancing Redox Reaction Redox Reactions Method Electrons

Difference Between Ion Electron Method And Oxidation Number Method Compare The Difference

Ion Electron Method 11th Chemistry Basic Chemistry Unit 1 In YouTube
V3 9
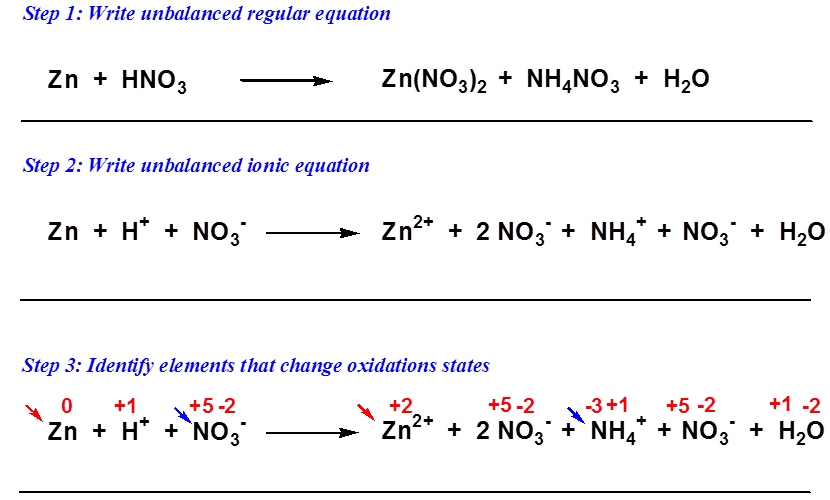
PPT Chapter 6 Oxidation Reduction Reactions PowerPoint Presentation ID 5980544
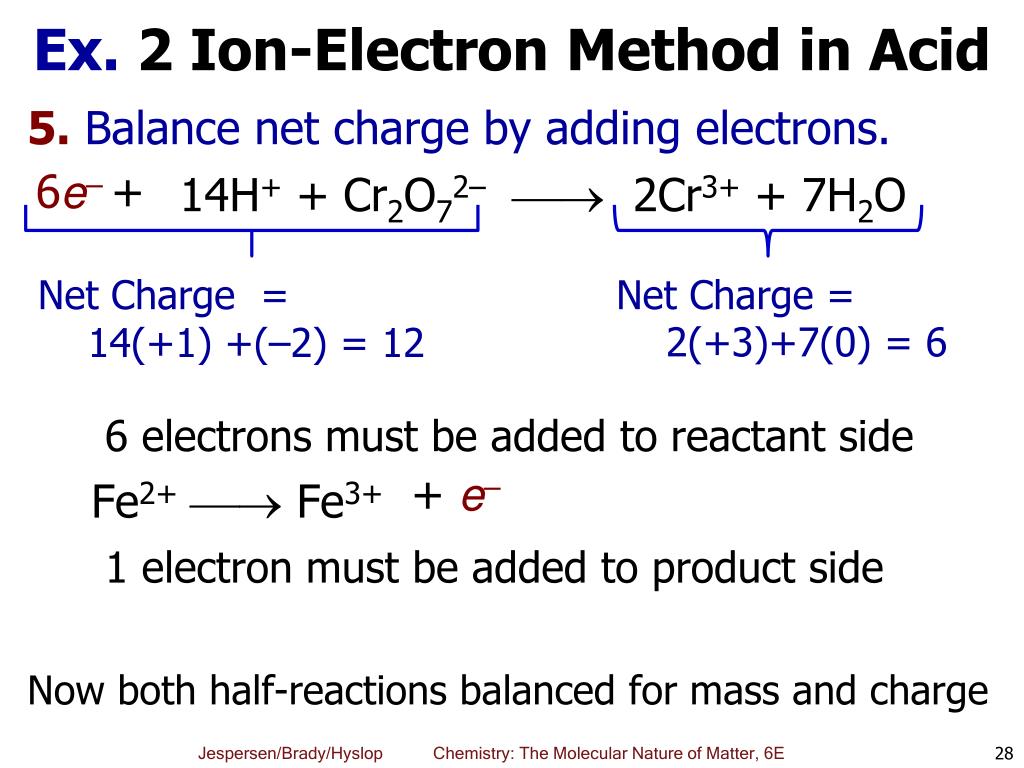
PPT Chapter 6 Oxidation Reduction Reactions PowerPoint Presentation ID 4012495

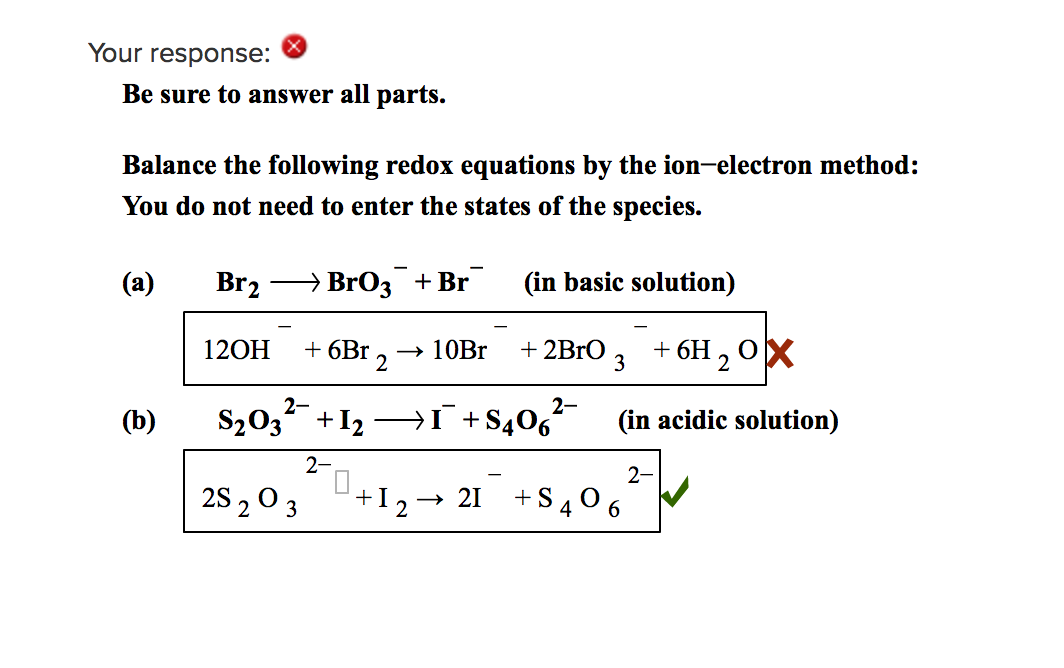
https://chem.libretexts.org/Courses/Saint_Marys...
Use the ion electron method to complete and balance the following skeletal redox equations occurring in either acidic or basic aqueous solution as indicated Identify the oxidation and reduction half reactions in each case In acidic aqueous solution Cu NO3 rightarrow Cu 2 N 2O 4
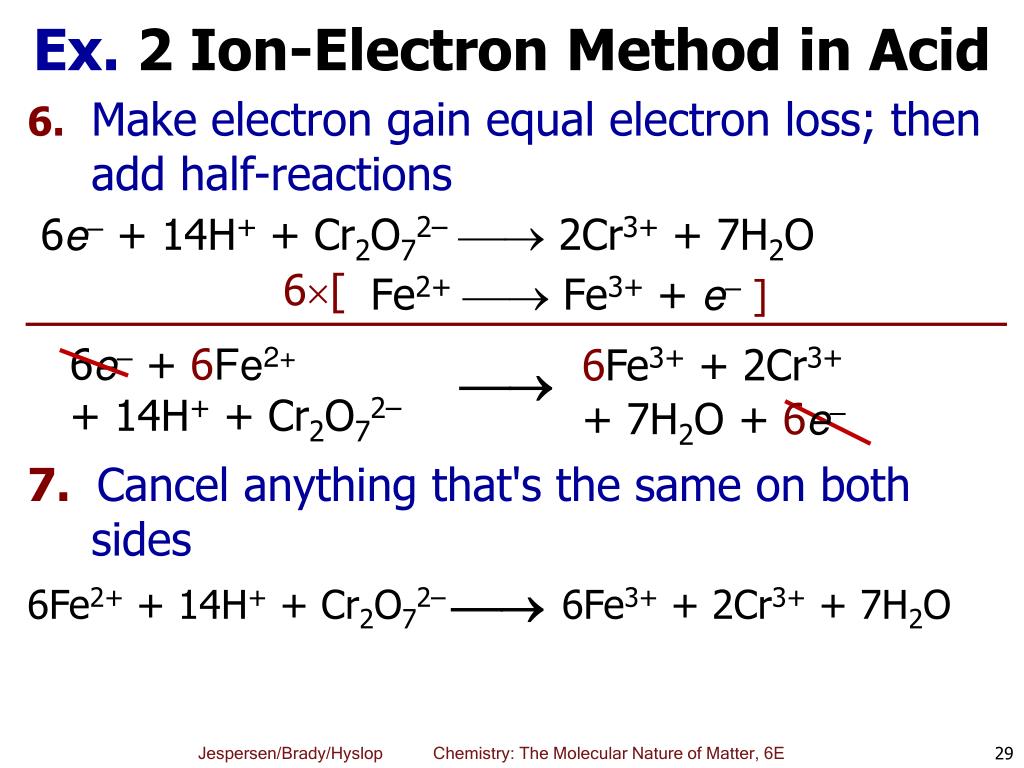
https://www.simply.science/images/content/...
In order to balance the complex oxidation reduction reactions within a length of time chemists have developed a simple and most versatile method known as Ion electron method The reactions which occur in acid solutions containing H ions can be balanced by using Ion electron method which in turn involves six steps Step 1
Use the ion electron method to complete and balance the following skeletal redox equations occurring in either acidic or basic aqueous solution as indicated Identify the oxidation and reduction half reactions in each case In acidic aqueous solution Cu NO3 rightarrow Cu 2 N 2O 4
In order to balance the complex oxidation reduction reactions within a length of time chemists have developed a simple and most versatile method known as Ion electron method The reactions which occur in acid solutions containing H ions can be balanced by using Ion electron method which in turn involves six steps Step 1

V3 9

Difference Between Ion Electron Method And Oxidation Number Method Compare The Difference

PPT Chapter 6 Oxidation Reduction Reactions PowerPoint Presentation ID 5980544

PPT Chapter 6 Oxidation Reduction Reactions PowerPoint Presentation ID 4012495
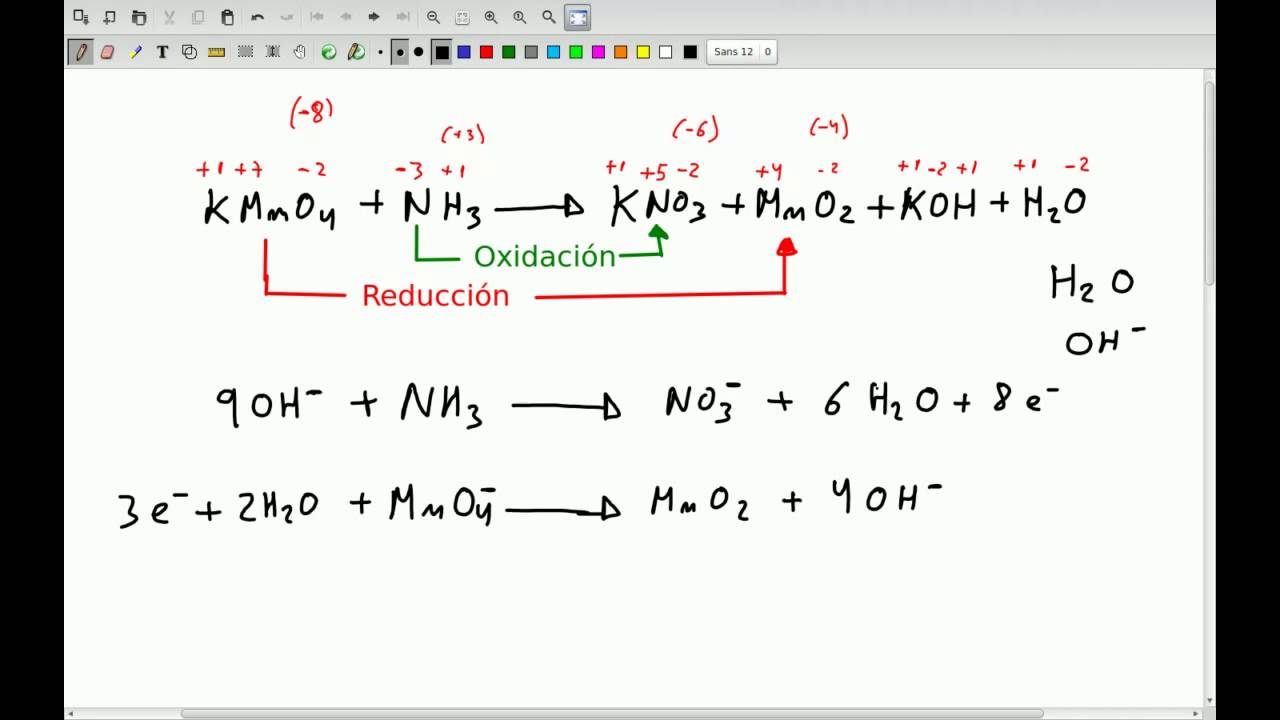
BALANCEO ION ELECTRON PDF

BALANCE REDOX EQUATION ION ELECTRON METHOD IN BASIC MEDIUM YouTube

BALANCE REDOX EQUATION ION ELECTRON METHOD IN BASIC MEDIUM YouTube

Difference Between Ion Electron Method And Oxidation Number Method Compare The Difference