In this age of technology, with screens dominating our lives and our lives are dominated by screens, the appeal of tangible printed objects hasn't waned. In the case of educational materials such as creative projects or just adding personal touches to your area, Ion Electron Method In Acidic Medium Examples can be an excellent resource. The following article is a take a dive into the sphere of "Ion Electron Method In Acidic Medium Examples," exploring their purpose, where they are available, and how they can add value to various aspects of your daily life.
Get Latest Ion Electron Method In Acidic Medium Examples Below

Ion Electron Method In Acidic Medium Examples
Ion Electron Method In Acidic Medium Examples - Ion Electron Method In Acidic Medium Examples, Ion Electron Method In Acidic Medium, Ion Electron Method Examples, What Is The Ion Electron Method, Ion Electron Method Class 11 Examples, Ion Electron Method In Basic Medium
Balance redox equations using the ion electron method in an acidic solutions There are two common techniques for balancing redox equations oxidation number change method ion
For reactions in basic medium first balance the atoms as you would for an acidic solution Then for each H ion add OH ion to both sides of the half reaction
Printables for free cover a broad collection of printable items that are available online at no cost. They are available in numerous types, like worksheets, templates, coloring pages, and much more. The value of Ion Electron Method In Acidic Medium Examples is in their variety and accessibility.
More of Ion Electron Method In Acidic Medium Examples
redox reactions Nainital JEE NEET Ion Electron Method In Acidic Medium Chemistry Class 11

redox reactions Nainital JEE NEET Ion Electron Method In Acidic Medium Chemistry Class 11
This is demonstrated in the acidic and basic solution examples Besides the general rules for neutral conditions additional rules must be applied for aqueous reactions in
Example 11 Balance the equation for the reaction of stannous ion with pertechnetate in acidic solution Products are stannic ion Sn 4 and technetium IV Tc 4 ions Solution 1 Net
The Ion Electron Method In Acidic Medium Examples have gained huge recognition for a variety of compelling motives:
-
Cost-Effective: They eliminate the necessity to purchase physical copies or costly software.
-
Individualization They can make printed materials to meet your requirements when it comes to designing invitations making your schedule, or even decorating your home.
-
Educational Value Education-related printables at no charge provide for students of all ages. This makes them an essential tool for parents and teachers.
-
Convenience: Instant access to the vast array of design and templates saves time and effort.
Where to Find more Ion Electron Method In Acidic Medium Examples
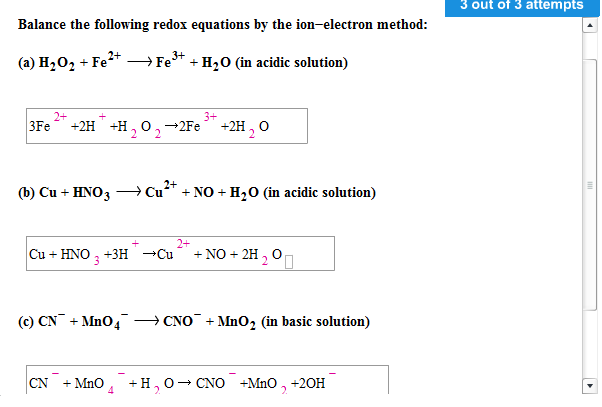
Balance The Following Redox Reactions By The Ion Electron Method In Acidic Medium MnO 4 aq

Balance The Following Redox Reactions By The Ion Electron Method In Acidic Medium MnO 4 aq
The reactions which occur in acid solutions containing H ions can be balanced by using Ion electron method which in turn involves six steps Step 1 In the first step the 2 reactants
The Half Equation Method is used to balance these reactions In a redox reaction one or more element becomes oxidized and one or more element becomes reduced
Now that we've piqued your interest in Ion Electron Method In Acidic Medium Examples Let's take a look at where the hidden treasures:
1. Online Repositories
- Websites such as Pinterest, Canva, and Etsy provide an extensive selection of Ion Electron Method In Acidic Medium Examples for various needs.
- Explore categories such as furniture, education, crafting, and organization.
2. Educational Platforms
- Forums and websites for education often offer free worksheets and worksheets for printing for flashcards, lessons, and worksheets. materials.
- The perfect resource for parents, teachers as well as students searching for supplementary sources.
3. Creative Blogs
- Many bloggers are willing to share their original designs and templates free of charge.
- The blogs covered cover a wide spectrum of interests, all the way from DIY projects to party planning.
Maximizing Ion Electron Method In Acidic Medium Examples
Here are some ways that you can make use use of printables for free:
1. Home Decor
- Print and frame stunning images, quotes, or seasonal decorations to adorn your living spaces.
2. Education
- Utilize free printable worksheets to build your knowledge at home as well as in the class.
3. Event Planning
- Design invitations, banners as well as decorations for special occasions like weddings or birthdays.
4. Organization
- Stay organized with printable calendars including to-do checklists, daily lists, and meal planners.
Conclusion
Ion Electron Method In Acidic Medium Examples are an abundance of fun and practical tools designed to meet a range of needs and desires. Their access and versatility makes them a fantastic addition to your professional and personal life. Explore the vast world that is Ion Electron Method In Acidic Medium Examples today, and unlock new possibilities!
Frequently Asked Questions (FAQs)
-
Are Ion Electron Method In Acidic Medium Examples truly completely free?
- Yes, they are! You can print and download these documents for free.
-
Can I make use of free printables for commercial purposes?
- It's dependent on the particular conditions of use. Always verify the guidelines of the creator before utilizing printables for commercial projects.
-
Do you have any copyright rights issues with printables that are free?
- Certain printables may be subject to restrictions in use. Make sure to read the terms and conditions provided by the author.
-
How do I print printables for free?
- You can print them at home using either a printer at home or in a print shop in your area for higher quality prints.
-
What program do I require to view printables for free?
- Most printables come with PDF formats, which can be opened with free software like Adobe Reader.
Balance The Following Redox Reactions By The Ion Electron Method In Acidic Medium MnO 4 aq

BALANCE REDOX EQUATION ION ELECTRON METHOD IN BASIC MEDIUM YouTube

Check more sample of Ion Electron Method In Acidic Medium Examples below
Balance The Following Redox Reactions By The Ion Electron Method In Acidic Medium MnO 4 aq

Ion Electron Method 11th Chemistry Basic Chemistry Unit 1 In YouTube
Solved Balance The Following Redox Equations By The Chegg
Balancing Redox Reaction By Ion Electron Method In Acidic Medium Ion Electron Method

Balance The Following Redox Reactions By The Ion Electron Method In Basis Medium MnO 4 aq

Balance Equation In Acidic Medium By Oxidation And Ion Electron Method Chemistry Redox


https://mi01000971.schoolwires.net/cms/lib...
For reactions in basic medium first balance the atoms as you would for an acidic solution Then for each H ion add OH ion to both sides of the half reaction

http://www.savitapall.com/Redox/Notes/Balancing by Ion...
2 Multiply each reaction by a suitable factor so that the electrons produced equals the electrons consumed i e electron gained equals electron lost 3 Add the two reactions
For reactions in basic medium first balance the atoms as you would for an acidic solution Then for each H ion add OH ion to both sides of the half reaction
2 Multiply each reaction by a suitable factor so that the electrons produced equals the electrons consumed i e electron gained equals electron lost 3 Add the two reactions

Balancing Redox Reaction By Ion Electron Method In Acidic Medium Ion Electron Method

Ion Electron Method 11th Chemistry Basic Chemistry Unit 1 In YouTube

Balance The Following Redox Reactions By The Ion Electron Method In Basis Medium MnO 4 aq

Balance Equation In Acidic Medium By Oxidation And Ion Electron Method Chemistry Redox

Balance The Following Redox Reactions By The Ion Electron Method In Acidic Medium MnO 4 aq

Balance The Following Redox Reactions By The Ion Electron Method In Acidic Medium MnO 4 aq

Balance The Following Redox Reactions By The Ion Electron Method In Acidic Medium MnO 4 aq

Balancing Of Equation By Ion Electron Method In Acidic Medium N i Cr2 O7