In this age of electronic devices, where screens dominate our lives and the appeal of physical printed materials isn't diminishing. It doesn't matter if it's for educational reasons such as creative projects or simply adding some personal flair to your space, How To Do Gas Stoichiometry are now an essential resource. Here, we'll take a dive into the world of "How To Do Gas Stoichiometry," exploring their purpose, where they can be found, and what they can do to improve different aspects of your daily life.
Get Latest How To Do Gas Stoichiometry Below
.PNG)
How To Do Gas Stoichiometry
How To Do Gas Stoichiometry - How To Do Gas Stoichiometry, How To Do Gas Stoichiometry At Stp, How To Do Gas Stoichiometry Not At Stp, How To Do Gas Stoichiometry Problems, How To Do Ideal Gas Law Stoichiometry, How Do You Do Gas Stoichiometry, What Is Gas Stoichiometry
Gas Stoichiometry Equations Part 1 To see all my Chemistry videos check out http socratic chemistry Examples and practice problems of solving equation stoichiometry questions with
If we know the volume pressure and temperature of a gas we can use the ideal gas equation to calculate how many moles of the gas are present If we know how many moles of a gas are involved we can calculate the volume of a gas at any temperature and pressure
Printables for free cover a broad selection of printable and downloadable content that can be downloaded from the internet at no cost. These printables come in different designs, including worksheets templates, coloring pages, and more. The appeal of printables for free is their flexibility and accessibility.
More of How To Do Gas Stoichiometry
Gas Stoichiometry Problems YouTube

Gas Stoichiometry Problems YouTube
Summary The ideal gas law relates the four independent physical properties of a gas at any time The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases Standard temperature and pressure STP are a useful set of benchmark conditions to compare other
This chemistry video tutorial explains how to solve gas stoichiometry problems at STP It covers the concept of molar volume and has plenty of practice problems associated with the gas laws
How To Do Gas Stoichiometry have garnered immense popularity because of a number of compelling causes:
-
Cost-Efficiency: They eliminate the need to purchase physical copies or costly software.
-
Flexible: The Customization feature lets you tailor printables to fit your particular needs be it designing invitations as well as organizing your calendar, or even decorating your house.
-
Educational Impact: Education-related printables at no charge offer a wide range of educational content for learners of all ages, which makes the perfect source for educators and parents.
-
Easy to use: Quick access to an array of designs and templates reduces time and effort.
Where to Find more How To Do Gas Stoichiometry
Gas Stoichiometry YouTube

Gas Stoichiometry YouTube
1 9K 107K views 4 years ago 1 product In this video I go over how to understand gas stoichiometry problems we ll go through common examples I typically see on Chemistry exams and how to know
We can tackle this stoichiometry problem using the following steps Step 1 Convert known reactant mass to moles In order to relate the amounts H A 2 SO A 4 and NaOH using a mole ratio we first need to know the quantity of H A 2 SO A 4
We hope we've stimulated your interest in printables for free Let's take a look at where you can find these treasures:
1. Online Repositories
- Websites like Pinterest, Canva, and Etsy provide a large collection and How To Do Gas Stoichiometry for a variety reasons.
- Explore categories like furniture, education, management, and craft.
2. Educational Platforms
- Educational websites and forums often offer worksheets with printables that are free along with flashcards, as well as other learning tools.
- Great for parents, teachers as well as students searching for supplementary resources.
3. Creative Blogs
- Many bloggers post their original designs and templates for no cost.
- These blogs cover a broad selection of subjects, from DIY projects to party planning.
Maximizing How To Do Gas Stoichiometry
Here are some inventive ways in order to maximize the use use of How To Do Gas Stoichiometry:
1. Home Decor
- Print and frame gorgeous art, quotes, or seasonal decorations to adorn your living spaces.
2. Education
- Print worksheets that are free for teaching at-home (or in the learning environment).
3. Event Planning
- Design invitations and banners as well as decorations for special occasions like weddings or birthdays.
4. Organization
- Keep track of your schedule with printable calendars as well as to-do lists and meal planners.
Conclusion
How To Do Gas Stoichiometry are a treasure trove of creative and practical resources catering to different needs and desires. Their availability and versatility make them an essential part of your professional and personal life. Explore the vast array of printables for free today and discover new possibilities!
Frequently Asked Questions (FAQs)
-
Are printables available for download really absolutely free?
- Yes, they are! You can download and print these resources at no cost.
-
Are there any free printables for commercial uses?
- It depends on the specific rules of usage. Always consult the author's guidelines before using any printables on commercial projects.
-
Do you have any copyright issues when you download printables that are free?
- Some printables may come with restrictions regarding usage. You should read the terms of service and conditions provided by the designer.
-
How can I print printables for free?
- You can print them at home with either a printer or go to a print shop in your area for premium prints.
-
What program must I use to open printables free of charge?
- Most PDF-based printables are available in the format of PDF, which can be opened using free programs like Adobe Reader.
Gas Stoichiometry Examples 1 2 YouTube

Ideal Gas Stoichiometry Volume To Volume Practice 1 YouTube
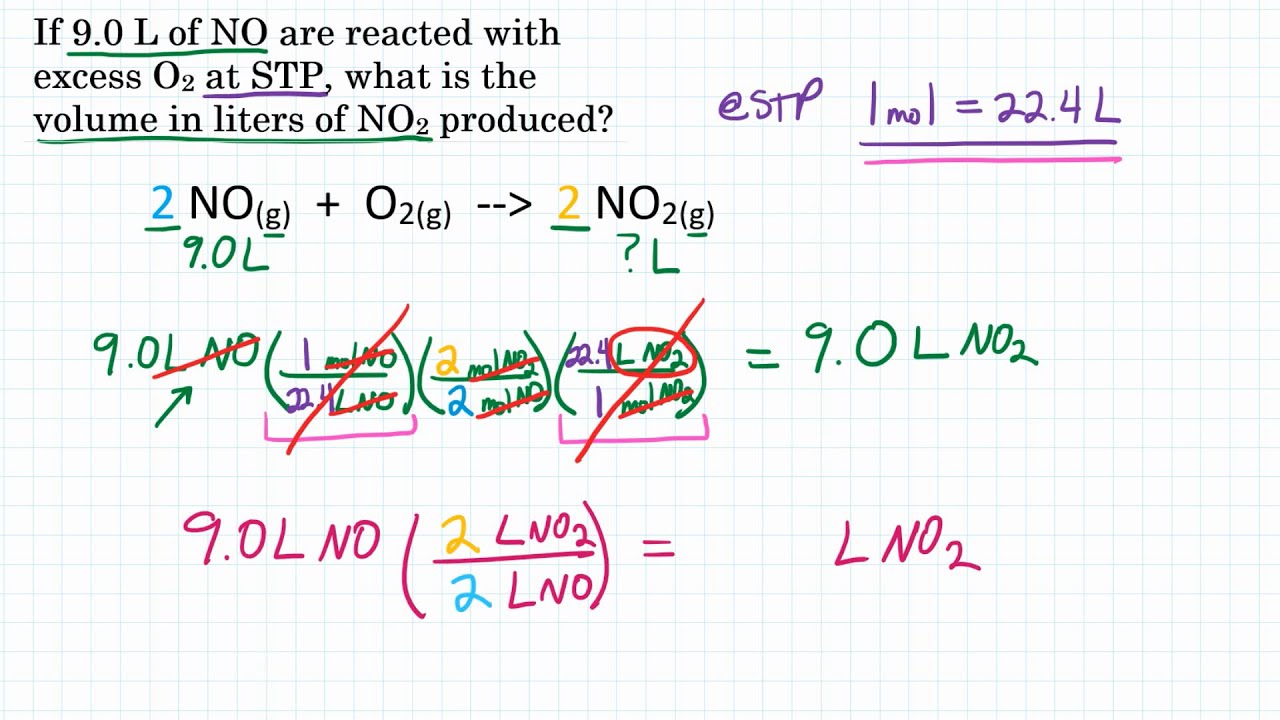
Check more sample of How To Do Gas Stoichiometry below
Gas Stoichiometry At STP Conditions Tutorial YouTube

Gas Law Stoichiometry YouTube
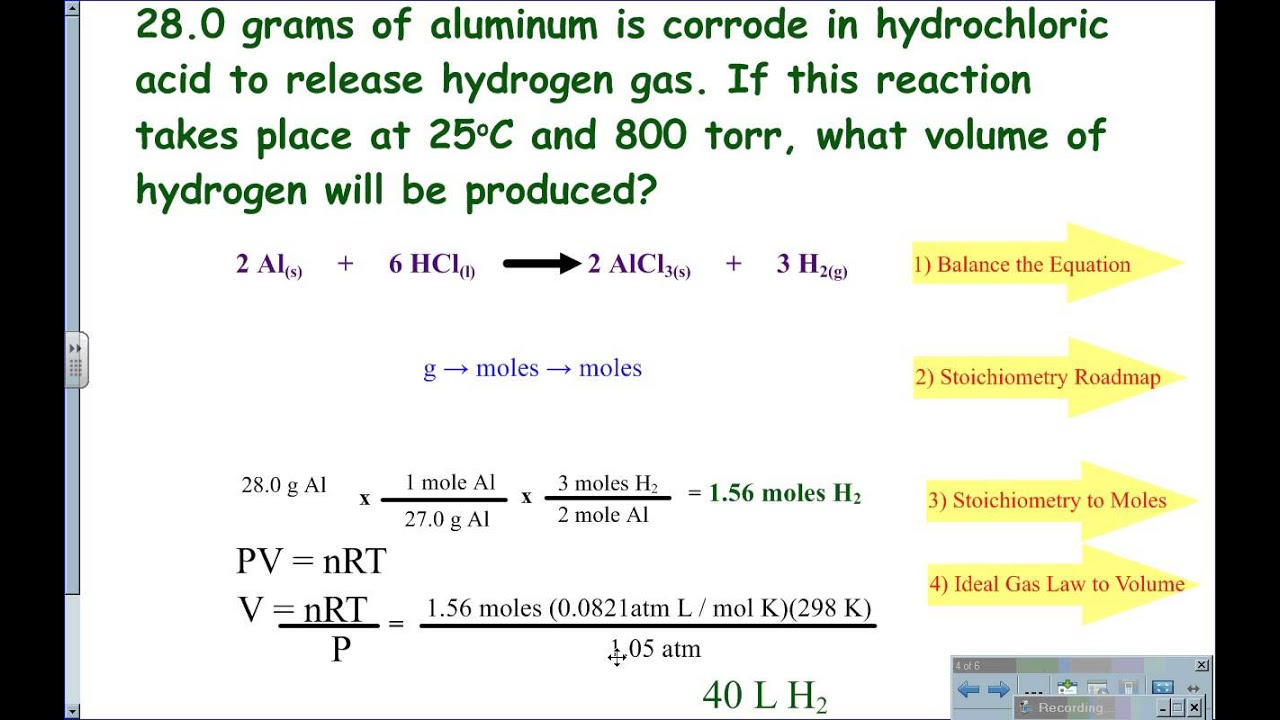
Gas Law Stoichiometry Not At STP YouTube

Gas Stoichiometry YouTube

Gas Stoichiometry Practice YouTube

Gas Stoichiometry YouTube

.PNG?w=186)
https://chem.libretexts.org/Courses/College_of_the...
If we know the volume pressure and temperature of a gas we can use the ideal gas equation to calculate how many moles of the gas are present If we know how many moles of a gas are involved we can calculate the volume of a gas at any temperature and pressure

https://www.wikihow.com/Do-Stoichiometry
If your reaction is happening at STP you can use 22 414 L mol to calculate the number of moles in a given volume of gas Divide the volume of gas L by the conversion factor to determine moles For example convert 3 2 liters of N 2 gas to moles 3 2 L 22 414 L mol 0 143 moles
If we know the volume pressure and temperature of a gas we can use the ideal gas equation to calculate how many moles of the gas are present If we know how many moles of a gas are involved we can calculate the volume of a gas at any temperature and pressure
If your reaction is happening at STP you can use 22 414 L mol to calculate the number of moles in a given volume of gas Divide the volume of gas L by the conversion factor to determine moles For example convert 3 2 liters of N 2 gas to moles 3 2 L 22 414 L mol 0 143 moles

Gas Stoichiometry YouTube

Gas Law Stoichiometry YouTube

Gas Stoichiometry Practice YouTube

Gas Stoichiometry YouTube
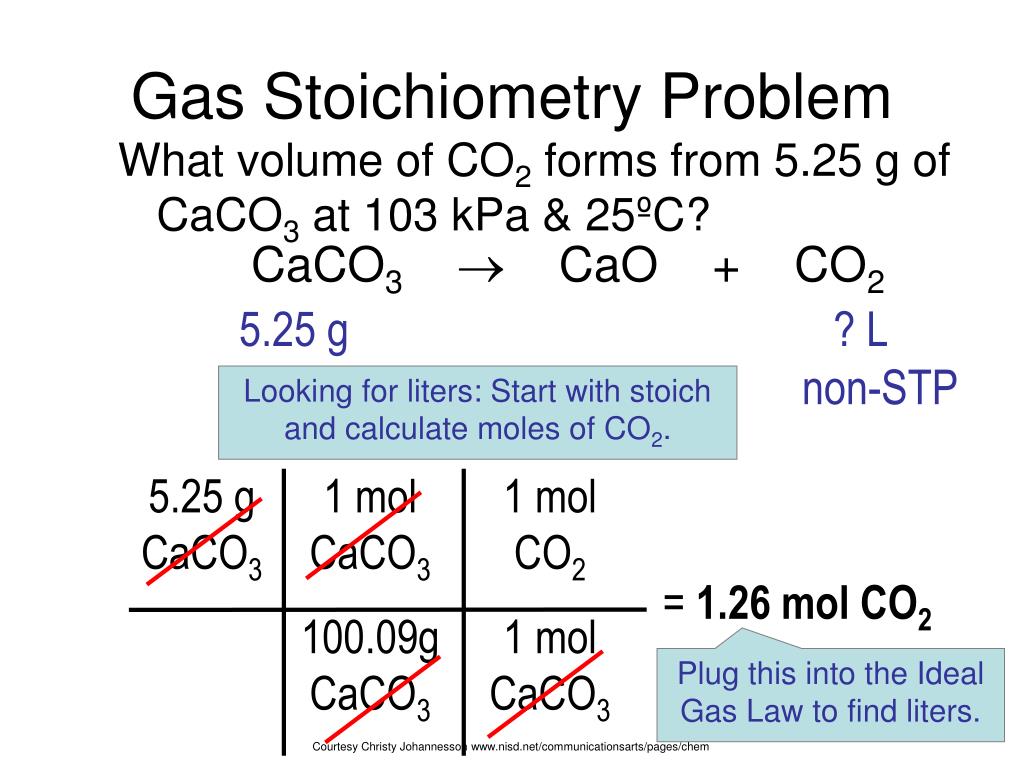
PPT Gas Stoichiometry PowerPoint Presentation Free Download ID 2956253

Gas Stoichiometry YouTube

Gas Stoichiometry YouTube

Gas Stoichiometry Problem 1 YouTube