In the digital age, with screens dominating our lives The appeal of tangible printed objects isn't diminished. In the case of educational materials for creative projects, simply to add personal touches to your space, Graham S Law Of Effusion Answer Key have become a valuable source. The following article is a dive to the depths of "Graham S Law Of Effusion Answer Key," exploring what they are, how to find them, and how they can improve various aspects of your life.
Get Latest Graham S Law Of Effusion Answer Key Below
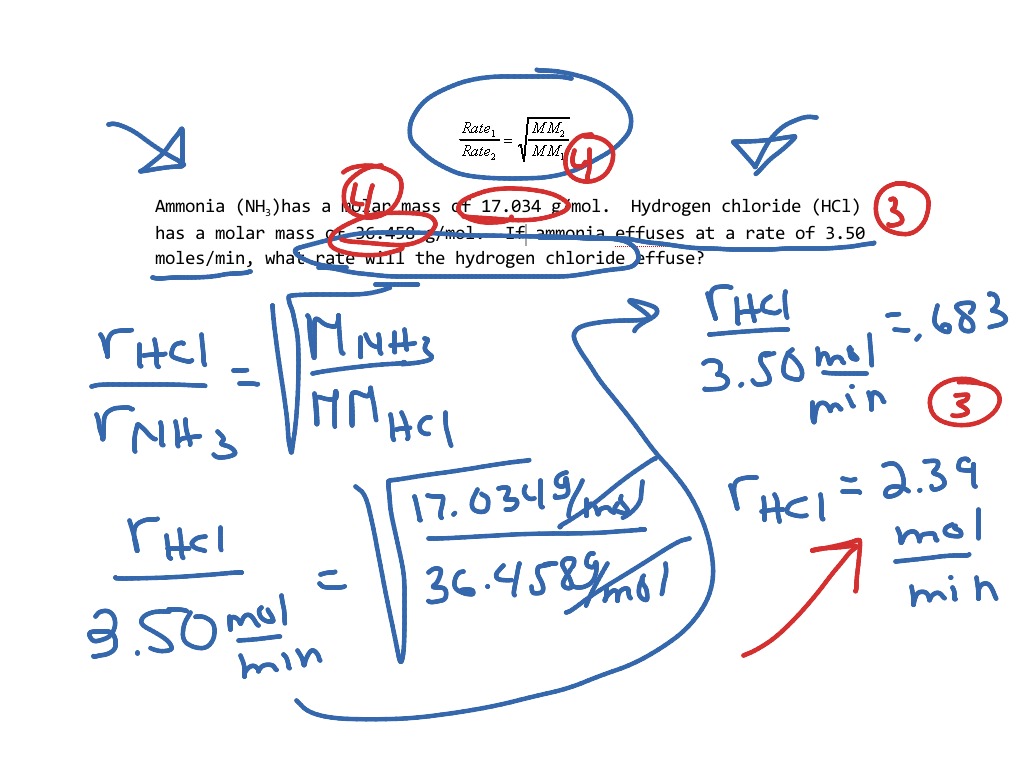
Graham S Law Of Effusion Answer Key
Graham S Law Of Effusion Answer Key - Graham's Law Of Effusion Answer Key, Graham's Law Of Effusion Worksheet Answers, Graham's Law Worksheet Answers, How Is Graham's Law Used In Everyday Life, Graham's Law States That
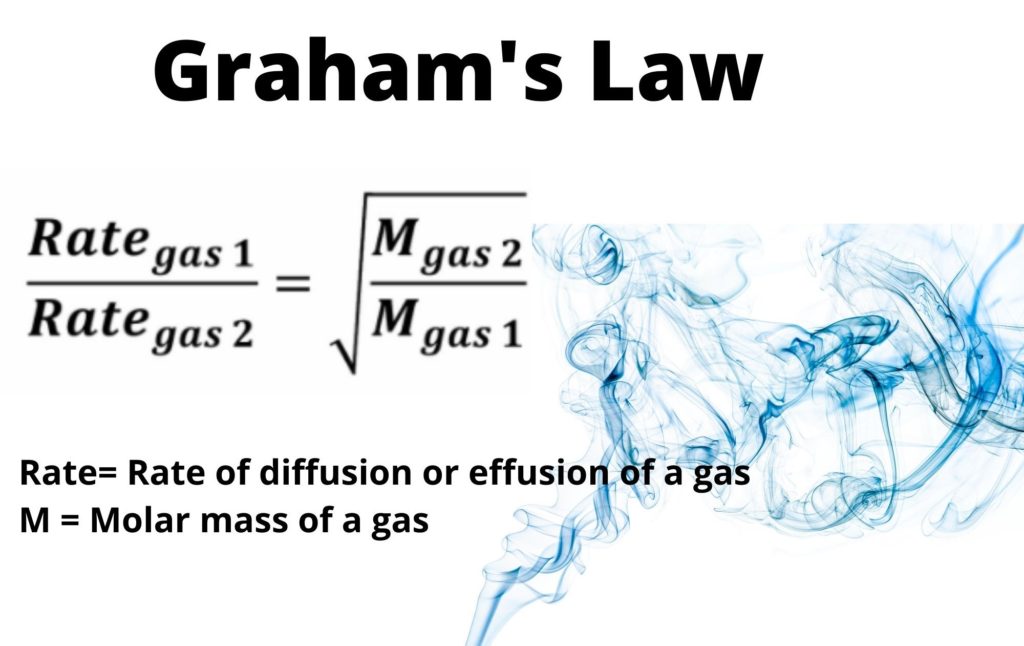
Graham s law states that the rate of diffusion or effusion of a gas is inversely proportional to the square root of its molar mass The Formula can be written as M1 is the molar mass of gas 1 M2 is the molar mass of gas 2 Rate1 is the
Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy Effusion refers to the movement of gas particles through a small hole Graham s Law states
Printables for free cover a broad range of printable, free items that are available online at no cost. They are available in numerous forms, like worksheets coloring pages, templates and more. The appeal of printables for free is in their variety and accessibility.
More of Graham S Law Of Effusion Answer Key
Chemistry 301 Graham s Law Of Effusion Graham s 19W Of EFFUSION

Chemistry 301 Graham s Law Of Effusion Graham s 19W Of EFFUSION
Graham s law states that the rate of effusion or diffusion of a gas is inversely proportional to the square root of the molar mass of the gas Graham s law can be understood
1K 89K views 3 years ago New AP General Chemistry Video Playlist This chemistry video tutorial provides a basic introduction into Graham s Law of Effusion It
Graham S Law Of Effusion Answer Key have garnered immense popularity because of a number of compelling causes:
-
Cost-Effective: They eliminate the need to purchase physical copies or expensive software.
-
Personalization The Customization feature lets you tailor printing templates to your own specific requirements be it designing invitations planning your schedule or even decorating your house.
-
Educational Use: Downloads of educational content for free offer a wide range of educational content for learners of all ages. This makes these printables a powerful tool for teachers and parents.
-
Convenience: Fast access various designs and templates reduces time and effort.
Where to Find more Graham S Law Of Effusion Answer Key
Graham s Law Of Diffusion Effusion

Graham s Law Of Diffusion Effusion
Chem 1210 with Matt Prater gases law of effusion worksheet under conditions in which the density of carbon dioxide is 1 96 and that of nitrogen gas is 1 25
Where Rate1 is the rate of effusion of the first gas volume or number of moles per unit time Rate2 is the rate of effusion for the second gas M1 is the molar mass of gas 1 M2 is the
We've now piqued your interest in Graham S Law Of Effusion Answer Key, let's explore where you can locate these hidden gems:
1. Online Repositories
- Websites such as Pinterest, Canva, and Etsy offer a huge selection of printables that are free for a variety of motives.
- Explore categories such as decorations for the home, education and organizing, and crafts.
2. Educational Platforms
- Educational websites and forums usually provide worksheets that can be printed for free or flashcards as well as learning tools.
- Great for parents, teachers and students looking for additional sources.
3. Creative Blogs
- Many bloggers provide their inventive designs and templates for no cost.
- The blogs are a vast selection of subjects, all the way from DIY projects to planning a party.
Maximizing Graham S Law Of Effusion Answer Key
Here are some fresh ways for you to get the best use of printables for free:
1. Home Decor
- Print and frame stunning artwork, quotes or other seasonal decorations to fill your living areas.
2. Education
- Print worksheets that are free for reinforcement of learning at home, or even in the classroom.
3. Event Planning
- Design invitations, banners, as well as decorations for special occasions like weddings and birthdays.
4. Organization
- Be organized by using printable calendars or to-do lists. meal planners.
Conclusion
Graham S Law Of Effusion Answer Key are an abundance with useful and creative ideas that cater to various needs and interests. Their access and versatility makes them a fantastic addition to every aspect of your life, both professional and personal. Explore the wide world of Graham S Law Of Effusion Answer Key today and discover new possibilities!
Frequently Asked Questions (FAQs)
-
Do printables with no cost really are they free?
- Yes, they are! You can download and print the resources for free.
-
Does it allow me to use free printables to make commercial products?
- It's determined by the specific usage guidelines. Always consult the author's guidelines before utilizing printables for commercial projects.
-
Do you have any copyright rights issues with printables that are free?
- Some printables may have restrictions in their usage. Be sure to review these terms and conditions as set out by the designer.
-
How can I print printables for free?
- You can print them at home using the printer, or go to a local print shop for the highest quality prints.
-
What software do I require to open printables for free?
- Many printables are offered in PDF format. These is open with no cost software such as Adobe Reader.
How To Solve Which Gas Will Effuse Faster Rate Of Effusion Graham s

Quiz Worksheet Graham s Law For Diffusion And Effusion Study

Check more sample of Graham S Law Of Effusion Answer Key below
LEC 9 Grahams Law Of Diffusion effusion gas Laws

Graham s Law Overview Calculation Expii

High School Chemistry Activity Graham s Law Of Effusion Learning Liftoff

Derivation Of Graham s Law Of Effusion Chemistry Gas Laws Science
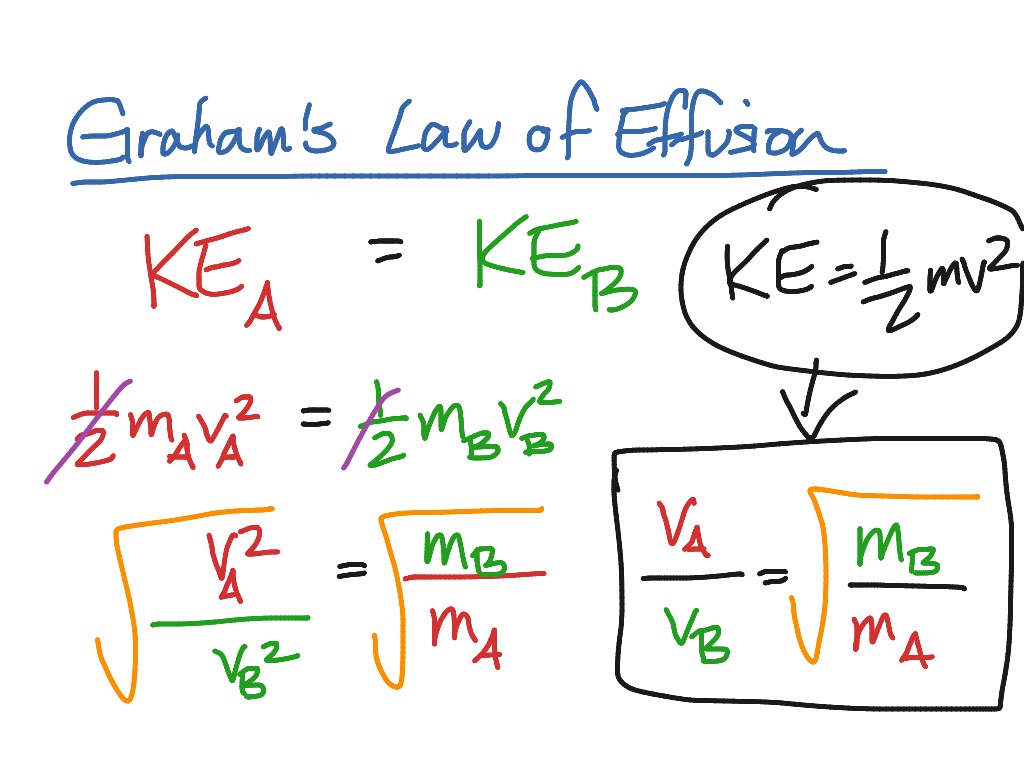
Graham s Law Of Diffusion Effusion And Its Derivation Chemistry Notes

Worksheet Graham s Law Of Effusion Quiz Quizizz
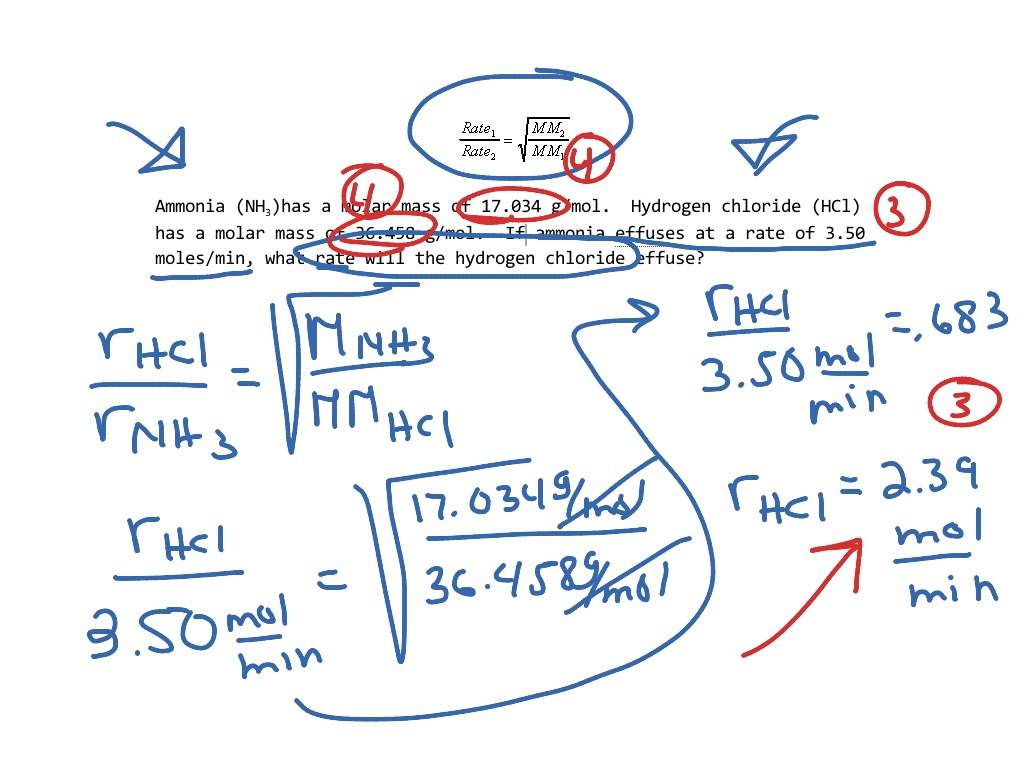
https://chem.libretexts.org/Bookshelves/Physical...
Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy Effusion refers to the movement of gas particles through a small hole Graham s Law states

https://www.chem.fsu.edu/.../chm1045/graham_key.pdf
Chemistry Graham s Law KEY Do the following problems showing your work and including all proper units 1 If neon gas travels at 400 m s at a given temperature calculate the velocity of
Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy Effusion refers to the movement of gas particles through a small hole Graham s Law states
Chemistry Graham s Law KEY Do the following problems showing your work and including all proper units 1 If neon gas travels at 400 m s at a given temperature calculate the velocity of

Derivation Of Graham s Law Of Effusion Chemistry Gas Laws Science

Graham s Law Overview Calculation Expii

Graham s Law Of Diffusion Effusion And Its Derivation Chemistry Notes
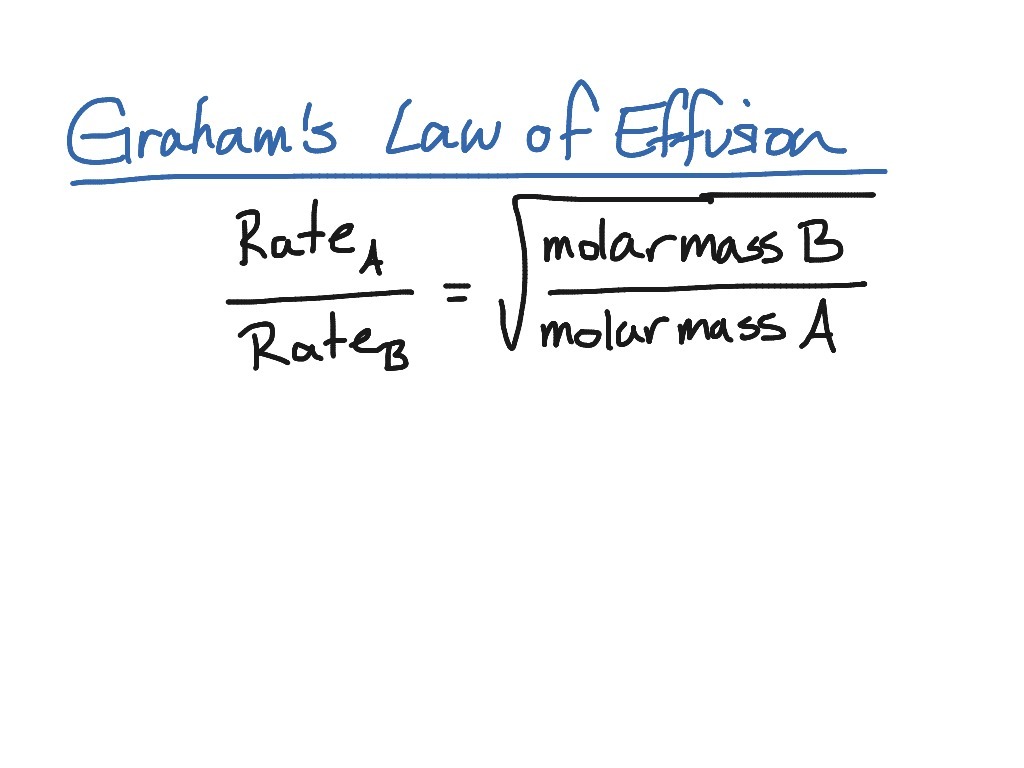
Worksheet Graham s Law Of Effusion Quiz Quizizz
Graham s Law Diffusion And Effusion Daily Life Examples What s Insight

Graham s Law Of Diffusion And Effusion YouTube

Graham s Law Of Diffusion And Effusion YouTube

Graham s Law Explained With Question Diffusion And Effusion YouTube