In a world in which screens are the norm and our lives are dominated by screens, the appeal of tangible printed items hasn't gone away. Whether it's for educational purposes in creative or artistic projects, or simply to add an element of personalization to your area, Why Are Nonpolar Covalent Compounds Not Soluble In Water are now a vital source. We'll take a dive in the world of "Why Are Nonpolar Covalent Compounds Not Soluble In Water," exploring their purpose, where you can find them, and how they can enhance various aspects of your daily life.
Get Latest Why Are Nonpolar Covalent Compounds Not Soluble In Water Below
Why Are Nonpolar Covalent Compounds Not Soluble In Water
Why Are Nonpolar Covalent Compounds Not Soluble In Water -
1 Oil has a density less than water so it just sits there on the top not spreading out into the more dense water below 2 Oil is very nonpolar meaning that a polar molecule like water doesn t get attracted to it Oil is
2 Answers Sorted by Many covalent molecules do dissociate in water HCl as pointed out in the comments phenol acetic acid for example whereas some
Printables for free cover a broad array of printable items that are available online at no cost. These resources come in various kinds, including worksheets coloring pages, templates and much more. The great thing about Why Are Nonpolar Covalent Compounds Not Soluble In Water lies in their versatility and accessibility.
More of Why Are Nonpolar Covalent Compounds Not Soluble In Water
Ms J s Chemistry Class Polar Vs NonPolar Covalent Bonds
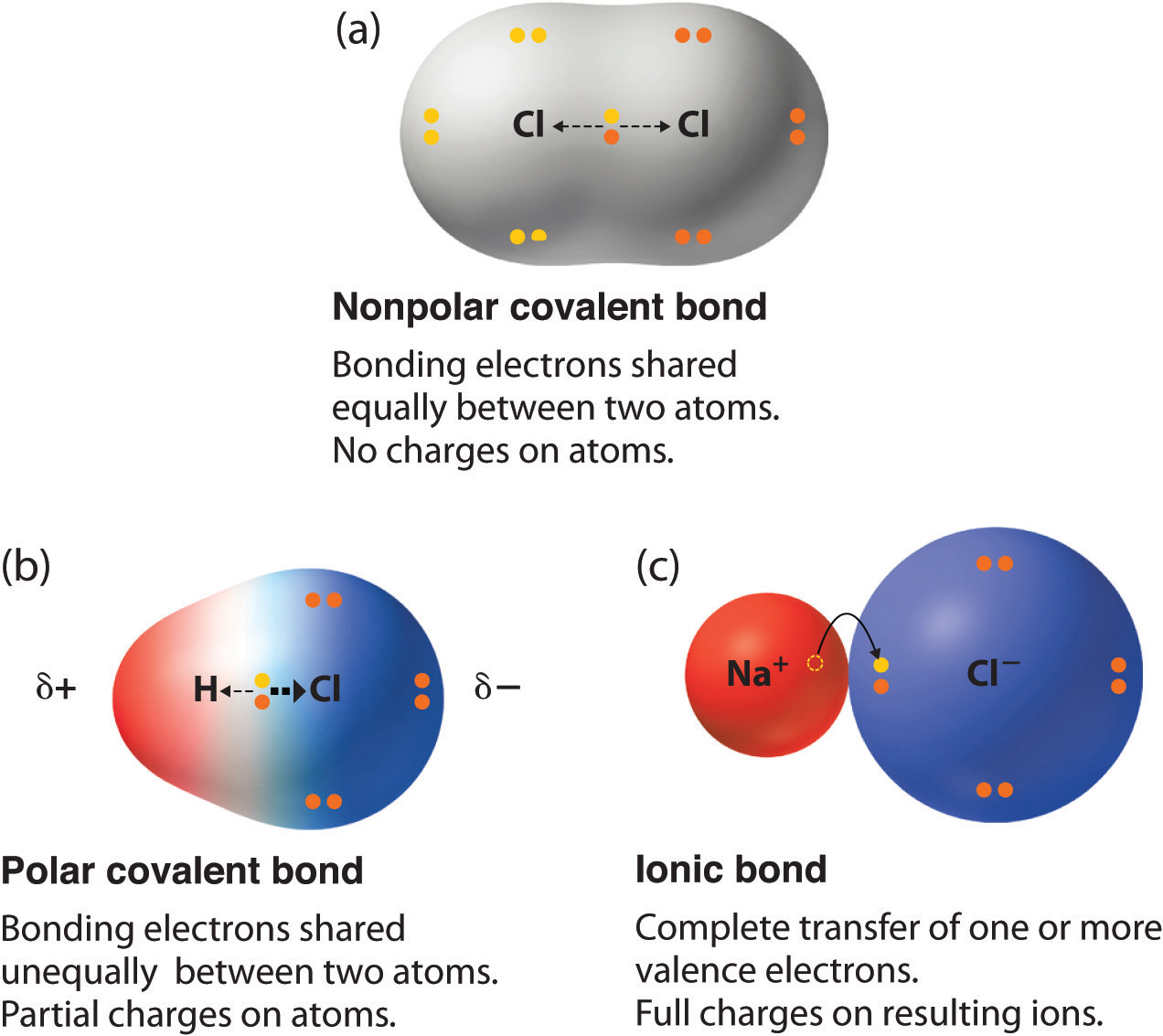
Ms J s Chemistry Class Polar Vs NonPolar Covalent Bonds
As the number of carbon atoms increases the solubility of the compound in water decreases For example hexanol
Biphenyl like sodium chloride is a colorless crystalline substance Biphenyl does not dissolve at all in water Why is this It is a very non polar molecule with only carbon carbon and carbon hydrogen bonds
Why Are Nonpolar Covalent Compounds Not Soluble In Water have gained a lot of popularity for several compelling reasons:
-
Cost-Efficiency: They eliminate the need to buy physical copies or costly software.
-
Individualization The Customization feature lets you tailor the design to meet your needs for invitations, whether that's creating them and schedules, or decorating your home.
-
Educational Use: Downloads of educational content for free offer a wide range of educational content for learners from all ages, making the perfect tool for parents and teachers.
-
An easy way to access HTML0: immediate access the vast array of design and templates reduces time and effort.
Where to Find more Why Are Nonpolar Covalent Compounds Not Soluble In Water
Nonpolar Covalent Bond Examples Thinking Latest
Nonpolar Covalent Bond Examples Thinking Latest
Insolubility in Water Many covalent compounds are nonpolar and are not soluble in water Water and ethanol are examples of polar covalent compounds that do dissolve ionic compounds and
We expect the solubility of nonpolar oxygen to be quite low in water and our answer confirms that In addition we can approximate 1 ppm O 2 as 1 mg O 2 L
We hope we've stimulated your interest in Why Are Nonpolar Covalent Compounds Not Soluble In Water we'll explore the places you can find these hidden treasures:
1. Online Repositories
- Websites like Pinterest, Canva, and Etsy provide a large collection and Why Are Nonpolar Covalent Compounds Not Soluble In Water for a variety objectives.
- Explore categories such as design, home decor, the arts, and more.
2. Educational Platforms
- Forums and websites for education often offer worksheets with printables that are free along with flashcards, as well as other learning materials.
- Ideal for parents, teachers, and students seeking supplemental sources.
3. Creative Blogs
- Many bloggers post their original designs and templates for free.
- The blogs covered cover a wide selection of subjects, everything from DIY projects to planning a party.
Maximizing Why Are Nonpolar Covalent Compounds Not Soluble In Water
Here are some unique ways create the maximum value of printables for free:
1. Home Decor
- Print and frame stunning artwork, quotes, or festive decorations to decorate your living areas.
2. Education
- Print free worksheets for teaching at-home also in the classes.
3. Event Planning
- Design invitations for banners, invitations and other decorations for special occasions like weddings and birthdays.
4. Organization
- Make sure you are organized with printable calendars for to-do list, lists of chores, and meal planners.
Conclusion
Why Are Nonpolar Covalent Compounds Not Soluble In Water are a treasure trove of innovative and useful resources that meet a variety of needs and hobbies. Their availability and versatility make they a beneficial addition to each day life. Explore the vast collection of Why Are Nonpolar Covalent Compounds Not Soluble In Water today and uncover new possibilities!
Frequently Asked Questions (FAQs)
-
Are Why Are Nonpolar Covalent Compounds Not Soluble In Water really free?
- Yes you can! You can download and print these documents for free.
-
Are there any free printables for commercial use?
- It's contingent upon the specific rules of usage. Always verify the guidelines of the creator before using their printables for commercial projects.
-
Do you have any copyright issues in printables that are free?
- Certain printables might have limitations on usage. Be sure to review these terms and conditions as set out by the creator.
-
How can I print Why Are Nonpolar Covalent Compounds Not Soluble In Water?
- You can print them at home with printing equipment or visit the local print shop for top quality prints.
-
What program will I need to access printables for free?
- The majority are printed in the PDF format, and can be opened with free programs like Adobe Reader.
Definition And Examples Of A Polar Bond
/PolarConvalentBond-58a715be3df78c345b77b57d.jpg)
Polar Covalent Bonds And Nonpolar Covalent Bonds Ionic Bonding Types
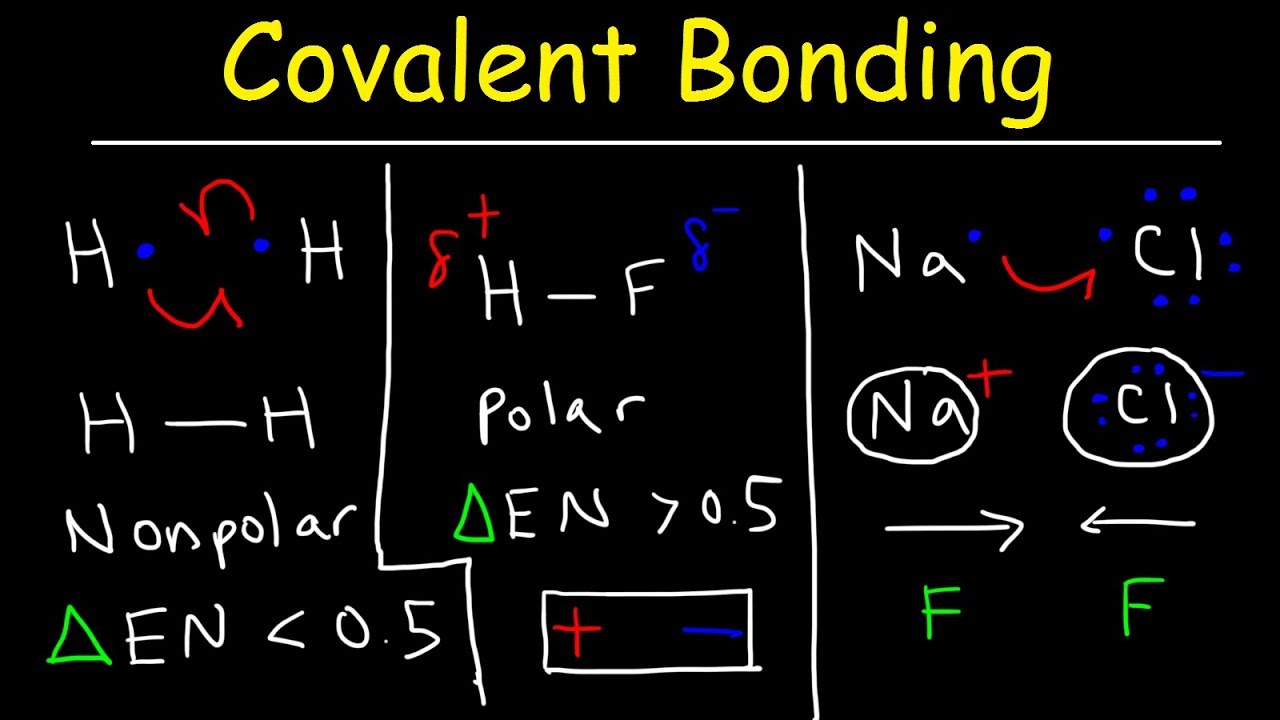
Check more sample of Why Are Nonpolar Covalent Compounds Not Soluble In Water below
Ionic Bonding Covalent Bonding Chemistry Quotes Atomic Structure

Organic Chemistry If All Intermolecular Forces Are Electrostatic In

Can A Fluorine Atom Ever Form A Nonpolar Covalent Bond
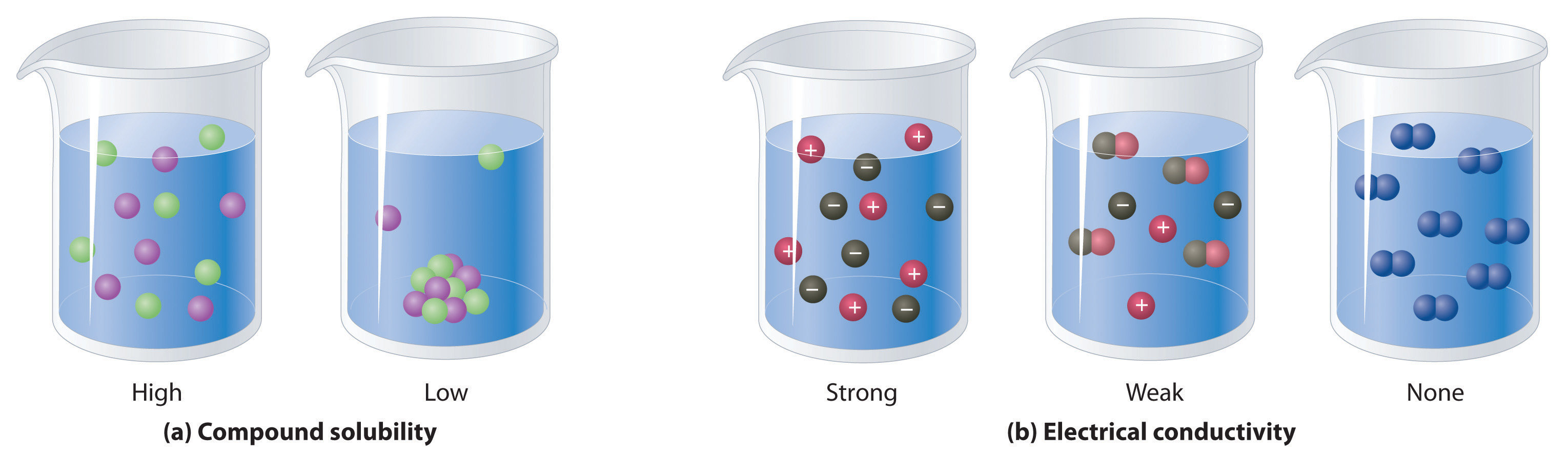
Aqueous Solutions
2 9 Atoms Isotopes Ions And Molecules Covalent Bonds And Other
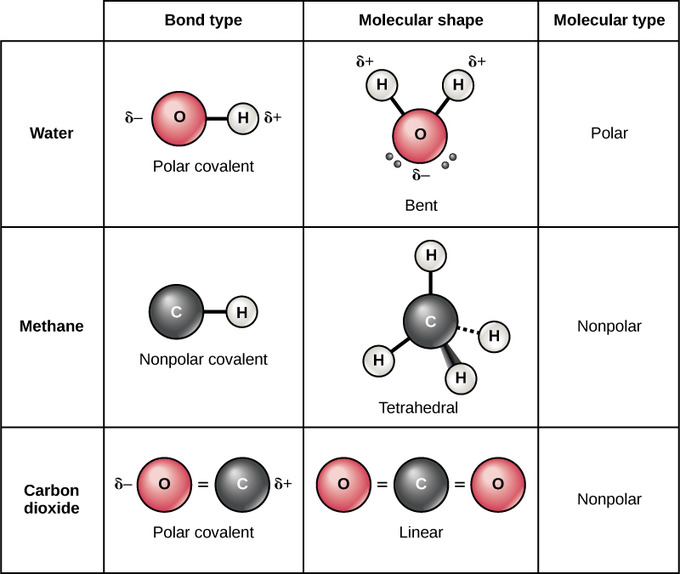
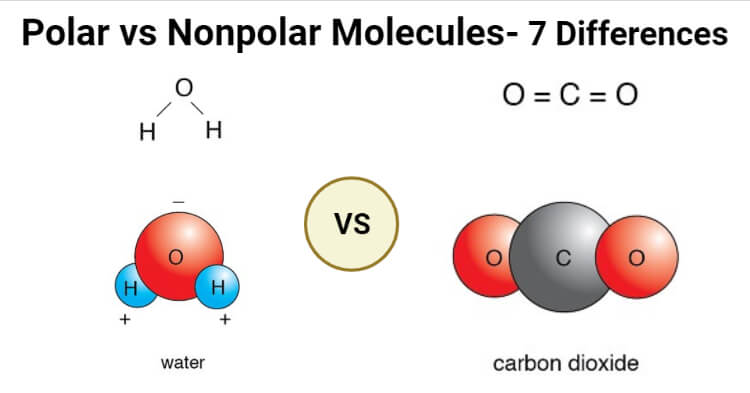
Polar And Nonpolar Molecules
https://chemistry.stackexchange.com/questions/57801
2 Answers Sorted by Many covalent molecules do dissociate in water HCl as pointed out in the comments phenol acetic acid for example whereas some
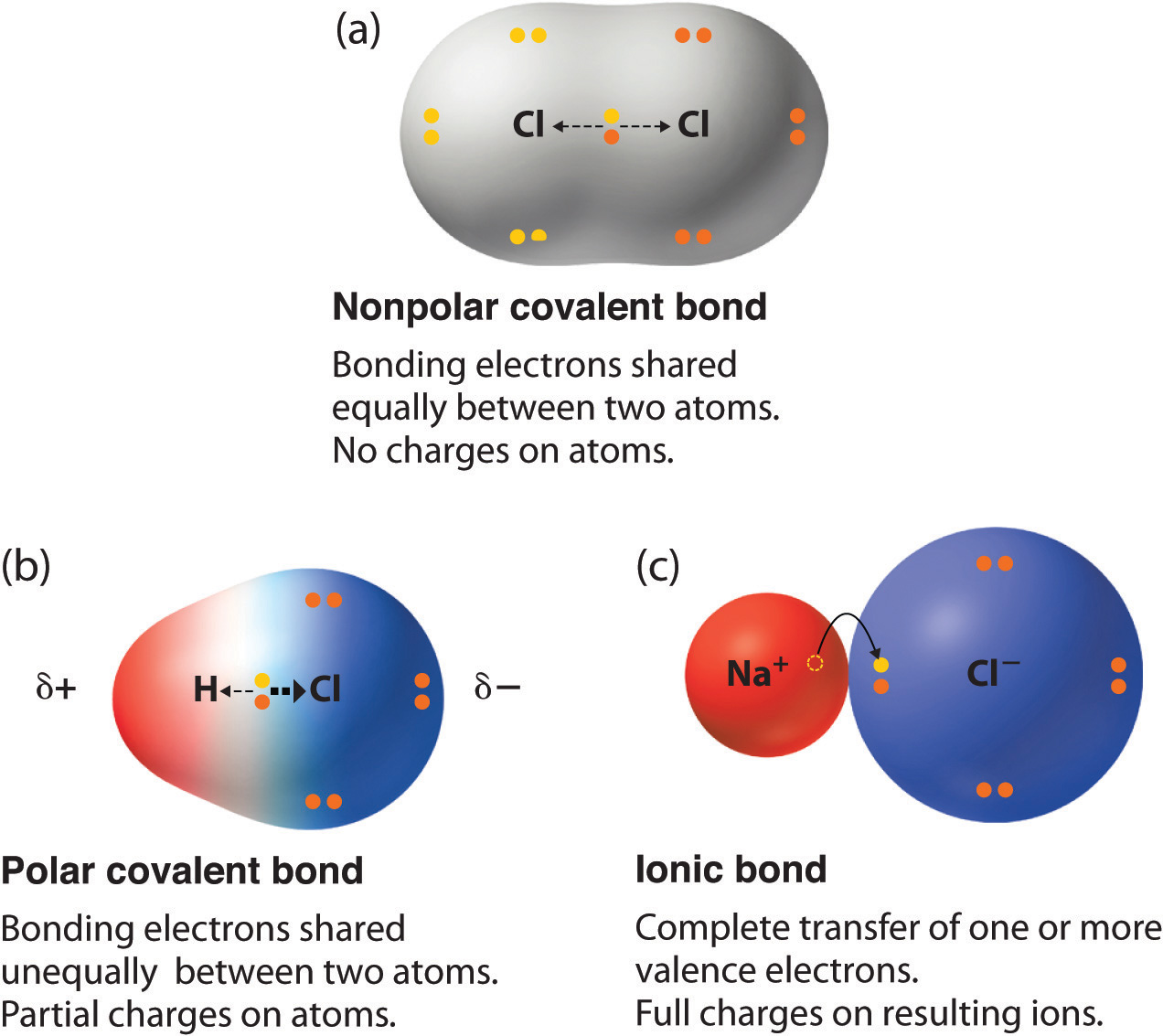
https://www.khanacademy.org/science/chemistry/...
Some non polar compounds do chemically interact with water because only a part of their molecule is non polar whilst another part is polar Ethanol is a good
2 Answers Sorted by Many covalent molecules do dissociate in water HCl as pointed out in the comments phenol acetic acid for example whereas some
Some non polar compounds do chemically interact with water because only a part of their molecule is non polar whilst another part is polar Ethanol is a good

Aqueous Solutions

Organic Chemistry If All Intermolecular Forces Are Electrostatic In

2 9 Atoms Isotopes Ions And Molecules Covalent Bonds And Other

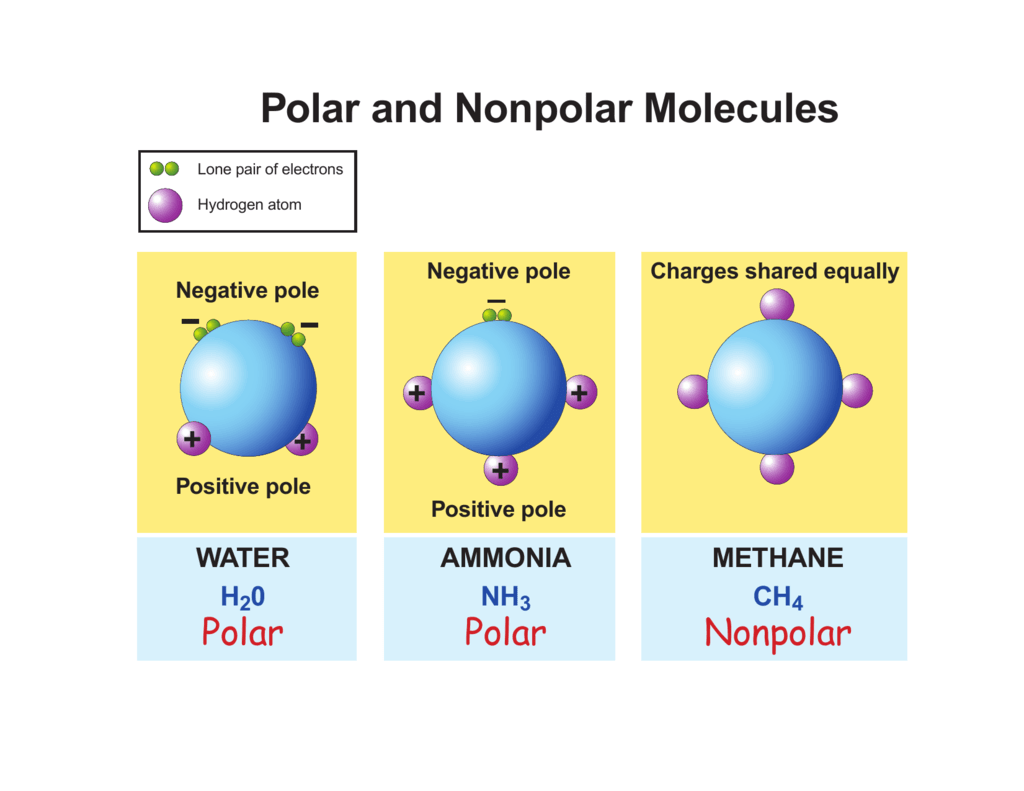
Polar And Nonpolar Molecules
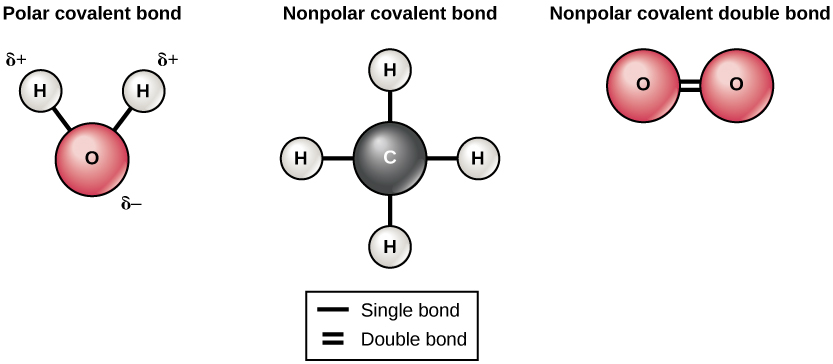
Covalent Bonds Biology I For Non Majors

Polar And Nonpolar Covalent Bonds Characteristics Differences

Polar And Nonpolar Covalent Bonds Characteristics Differences
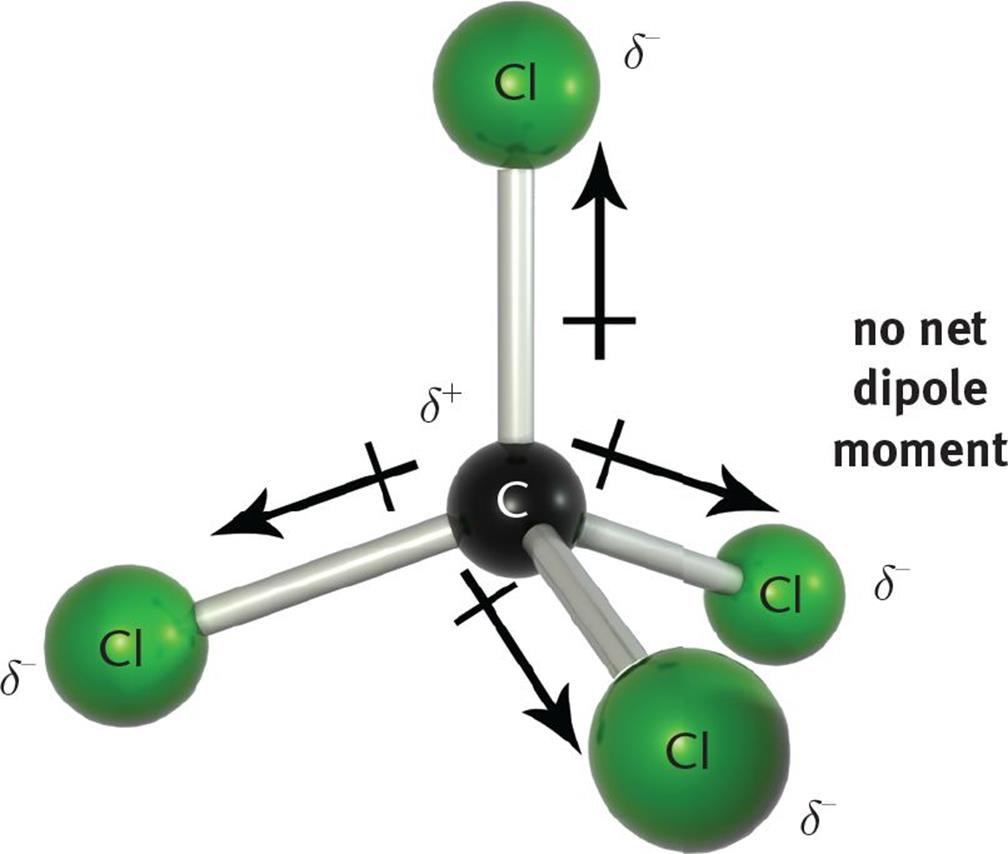
Figure 3 10 CCl 4 Is A Nonpolar Compound With Four Polar Bonds