In a world with screens dominating our lives and the appeal of physical printed materials hasn't faded away. For educational purposes in creative or artistic projects, or just adding personal touches to your home, printables for free are now a vital resource. Here, we'll dive deep into the realm of "What Is Dynamic Equilibrium Class 11th," exploring the benefits of them, where they are, and the ways that they can benefit different aspects of your daily life.
Get Latest What Is Dynamic Equilibrium Class 11th Below

What Is Dynamic Equilibrium Class 11th
What Is Dynamic Equilibrium Class 11th -
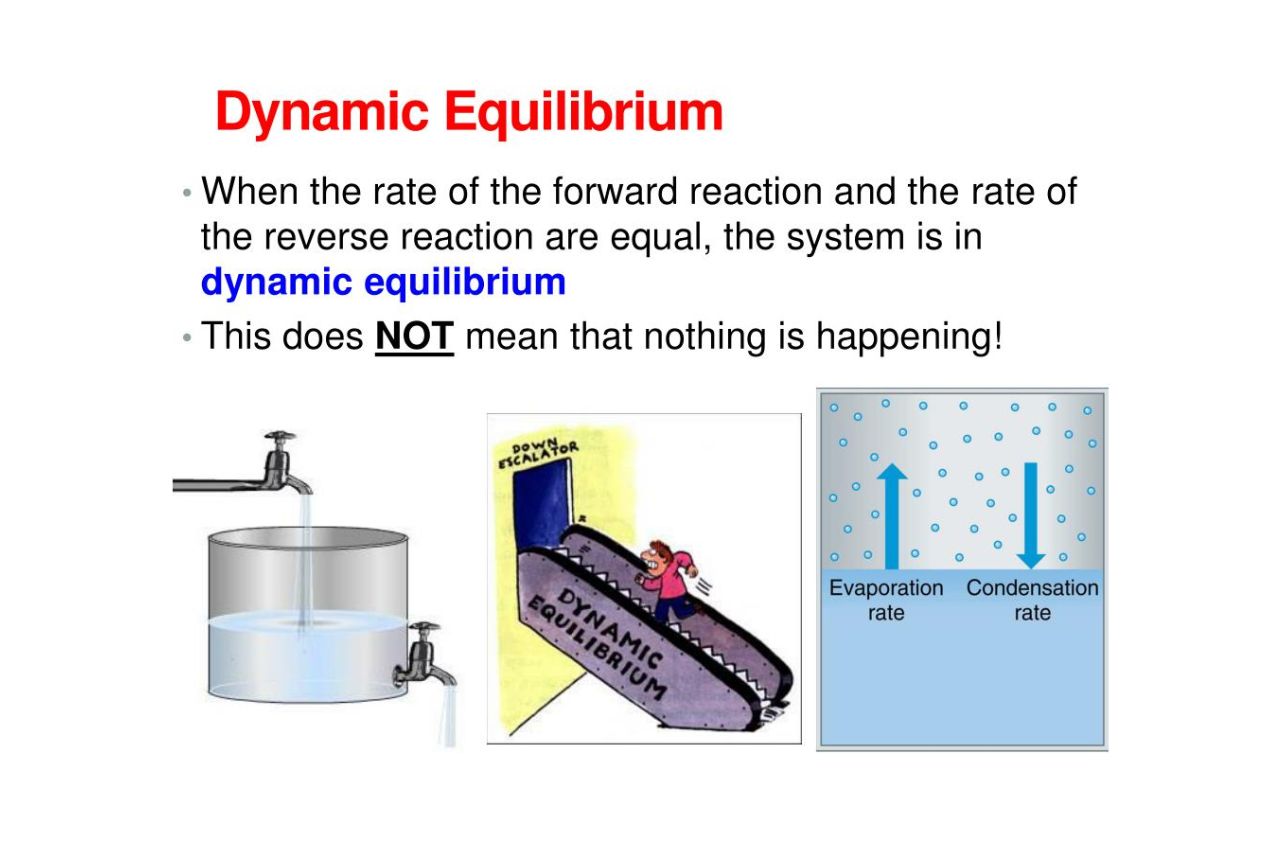
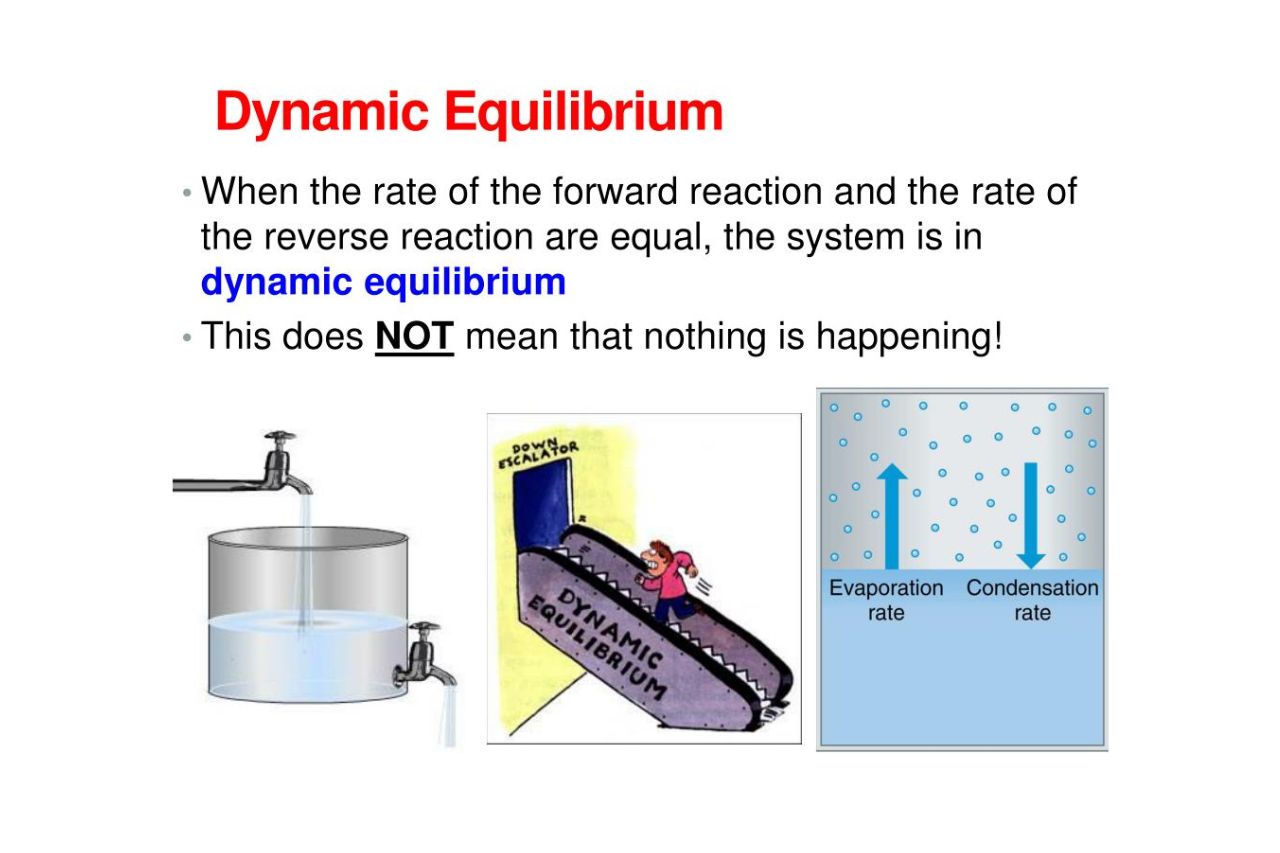
Dynamic equilibrium Google Classroom About Transcript Many physical and chemical processes are reversible A reversible process is said to be in dynamic equilibrium when the forward and reverse processes occur at the same rate resulting in no observable change in the system
Question What is dynamic equilibrium Solution A system in a steady state since forward reaction and backward reaction occur at the same rate In a dynamic equilibrium the rate of loss is equal to the rate of gain Dynamic equilibrium is applied in thermodynamics for systems involving reversible reactions Suggest
The What Is Dynamic Equilibrium Class 11th are a huge range of downloadable, printable documents that can be downloaded online at no cost. These printables come in different designs, including worksheets templates, coloring pages, and more. The appeal of printables for free lies in their versatility and accessibility.
More of What Is Dynamic Equilibrium Class 11th
18 Unbelievable Facts About Dynamic Equilibrium Facts
18 Unbelievable Facts About Dynamic Equilibrium Facts
Dynamic equilibrium is an important concept in chemistry But what is dynamic equilibrium exactly How can something be dynamic but also at equilibrium Keep reading to learn the best dynamic equilibrium definition common dynamic equilibrium examples and how dynamic and static equilibrium may look the
The system here is in dynamic equilibrium and we can infer the following i Both the opposing processes occur simultaneously ii Both the processes occur at the same rate so that the amount of ice and water
The What Is Dynamic Equilibrium Class 11th have gained huge popularity due to several compelling reasons:
-
Cost-Effective: They eliminate the need to buy physical copies or expensive software.
-
customization: There is the possibility of tailoring printing templates to your own specific requirements be it designing invitations to organize your schedule or even decorating your home.
-
Educational value: Printing educational materials for no cost are designed to appeal to students of all ages, making them a vital source for educators and parents.
-
An easy way to access HTML0: immediate access an array of designs and templates saves time and effort.
Where to Find more What Is Dynamic Equilibrium Class 11th
Dynamic Equilibrium Definition Important Examples
Dynamic Equilibrium Definition Important Examples
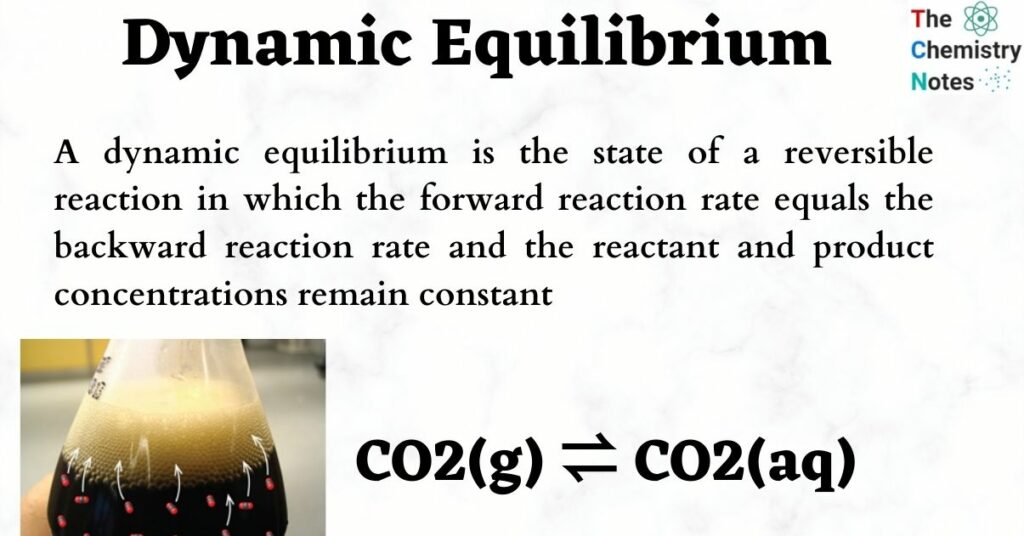
Chemical equilibrium refers to a state in which neither the reactants nor the products can alter any further In this situation the forward and backward reaction rates stay constant For students to grasp advanced concepts the concept of equilibrium becomes crucial Notes for Class 11 Chemistry chapter 7 provide
Free NCERT Solutions for Class 11 Chemistry Chapter 7 Equilibrium solved by expert teachers from latest edition books and as per NCERT CBSE guidelines Class 11 Chemistry Equilibrium NCERT Solutions and Extra Questions with Solutions to help you to revise complete Syllabus and Score More marks
Now that we've ignited your interest in printables for free We'll take a look around to see where they are hidden gems:
1. Online Repositories
- Websites such as Pinterest, Canva, and Etsy provide an extensive selection of What Is Dynamic Equilibrium Class 11th to suit a variety of objectives.
- Explore categories like design, home decor, craft, and organization.
2. Educational Platforms
- Educational websites and forums frequently offer free worksheets and worksheets for printing or flashcards as well as learning tools.
- It is ideal for teachers, parents as well as students searching for supplementary resources.
3. Creative Blogs
- Many bloggers are willing to share their original designs and templates at no cost.
- These blogs cover a wide array of topics, ranging ranging from DIY projects to party planning.
Maximizing What Is Dynamic Equilibrium Class 11th
Here are some unique ways create the maximum value of What Is Dynamic Equilibrium Class 11th:
1. Home Decor
- Print and frame beautiful images, quotes, or seasonal decorations that will adorn your living spaces.
2. Education
- Use printable worksheets for free for teaching at-home as well as in the class.
3. Event Planning
- Design invitations, banners as well as decorations for special occasions like birthdays and weddings.
4. Organization
- Make sure you are organized with printable calendars, to-do lists, and meal planners.
Conclusion
What Is Dynamic Equilibrium Class 11th are a treasure trove of fun and practical tools that satisfy a wide range of requirements and interests. Their accessibility and flexibility make they a beneficial addition to the professional and personal lives of both. Explore the world of What Is Dynamic Equilibrium Class 11th right now and open up new possibilities!
Frequently Asked Questions (FAQs)
-
Are What Is Dynamic Equilibrium Class 11th truly cost-free?
- Yes you can! You can print and download these files for free.
-
Are there any free printing templates for commercial purposes?
- It's based on the conditions of use. Always verify the guidelines provided by the creator prior to printing printables for commercial projects.
-
Do you have any copyright issues with What Is Dynamic Equilibrium Class 11th?
- Certain printables might have limitations in their usage. Always read the conditions and terms of use provided by the creator.
-
How do I print printables for free?
- You can print them at home using the printer, or go to a local print shop for more high-quality prints.
-
What software do I need in order to open printables that are free?
- The majority of PDF documents are provided in PDF format, which can be opened with free software like Adobe Reader.
Dynamics Equilibrium With Examples Physics Tutorials

Equilibrium Of Laws Of Motion In Physics Class 11

Check more sample of What Is Dynamic Equilibrium Class 11th below
What Is Dynamic Equilibrium Definition And Examples

Dynamic Nature Of Equilibrium Sri Chaitanya Infinity Learn Best
7 Important Difference Between Static And Dynamic Equilibrium With

Chemical Equilibrium I Types Of Equilibrium Equilibrium Constant

Msmcgartland licensed For Non commercial Use Only Chemical Systems

Why Is Dynamic Used To Describe Chemical Equilibrium


https://byjus.com/question-answer/what-is-dynamic-equilibrium
Question What is dynamic equilibrium Solution A system in a steady state since forward reaction and backward reaction occur at the same rate In a dynamic equilibrium the rate of loss is equal to the rate of gain Dynamic equilibrium is applied in thermodynamics for systems involving reversible reactions Suggest
https://www.learncbse.in/equilibrium-cbse-notes-class-11-chemistry
iii The equilibrium is dynamic since both the forward and backward processes occur at same rate iv At equilibrium the concentrations of substances become constant at constant temperature v The value of equilibrium constant represents the extent to which the process proceeds before equilibrium is achieved
Question What is dynamic equilibrium Solution A system in a steady state since forward reaction and backward reaction occur at the same rate In a dynamic equilibrium the rate of loss is equal to the rate of gain Dynamic equilibrium is applied in thermodynamics for systems involving reversible reactions Suggest
iii The equilibrium is dynamic since both the forward and backward processes occur at same rate iv At equilibrium the concentrations of substances become constant at constant temperature v The value of equilibrium constant represents the extent to which the process proceeds before equilibrium is achieved

Chemical Equilibrium I Types Of Equilibrium Equilibrium Constant

Dynamic Nature Of Equilibrium Sri Chaitanya Infinity Learn Best

Msmcgartland licensed For Non commercial Use Only Chemical Systems

Why Is Dynamic Used To Describe Chemical Equilibrium

7 1 Dynamic Equilibrium SL YouTube

Difference Between Static And Dynamic Equilibrium Equilibrium

Difference Between Static And Dynamic Equilibrium Equilibrium

Difference Between Chemical Equilibrium And Dynamic Equilibrium