In this age of electronic devices, in which screens are the norm yet the appeal of tangible printed objects isn't diminished. Be it for educational use and creative work, or simply adding personal touches to your area, What Is A Limiting Reactant In Stoichiometry have become an invaluable resource. We'll take a dive to the depths of "What Is A Limiting Reactant In Stoichiometry," exploring the benefits of them, where to find them and ways they can help you improve many aspects of your lives.
Get Latest What Is A Limiting Reactant In Stoichiometry Below
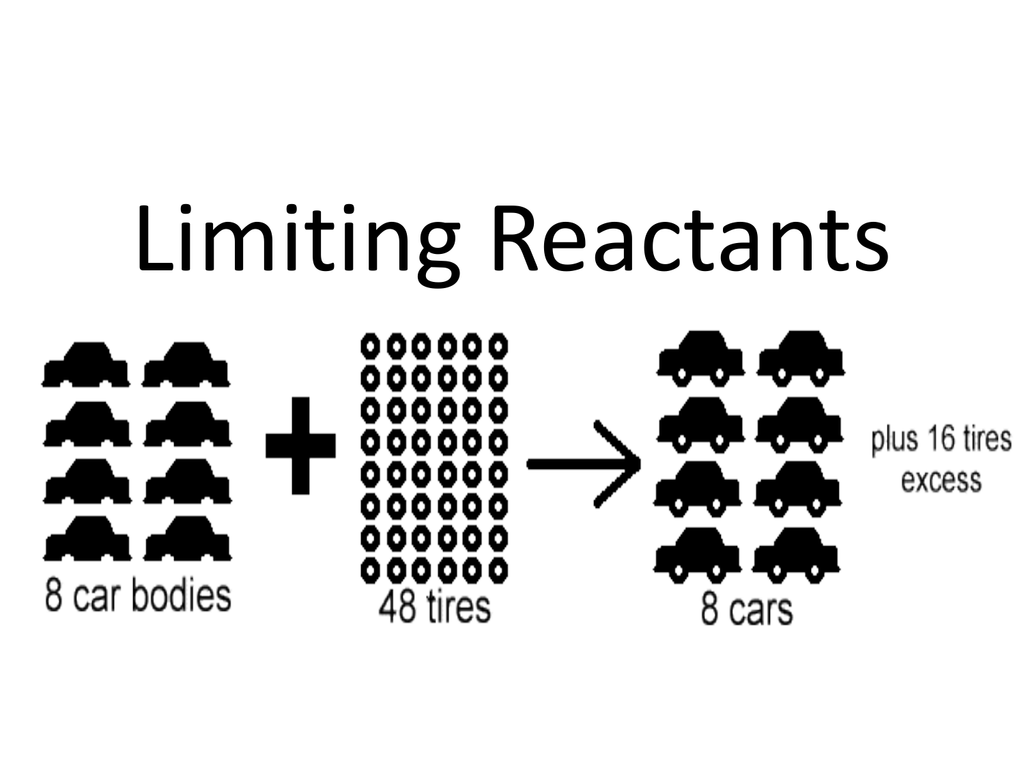
What Is A Limiting Reactant In Stoichiometry
What Is A Limiting Reactant In Stoichiometry -
The reactant which gets consumed and limits the amount of product formed is called the limiting reagent When a chemist carries out a reaction the reactants are not usually present in exact
Identify the limiting reactant limiting reagent in a given chemical reaction Calculate how much product will be produced from the limiting reactant Calculate how much reactant s remains
What Is A Limiting Reactant In Stoichiometry encompass a wide range of downloadable, printable documents that can be downloaded online at no cost. These resources come in various designs, including worksheets templates, coloring pages, and more. The value of What Is A Limiting Reactant In Stoichiometry is their versatility and accessibility.
More of What Is A Limiting Reactant In Stoichiometry
Limiting Reactant Definition Easy To Understand
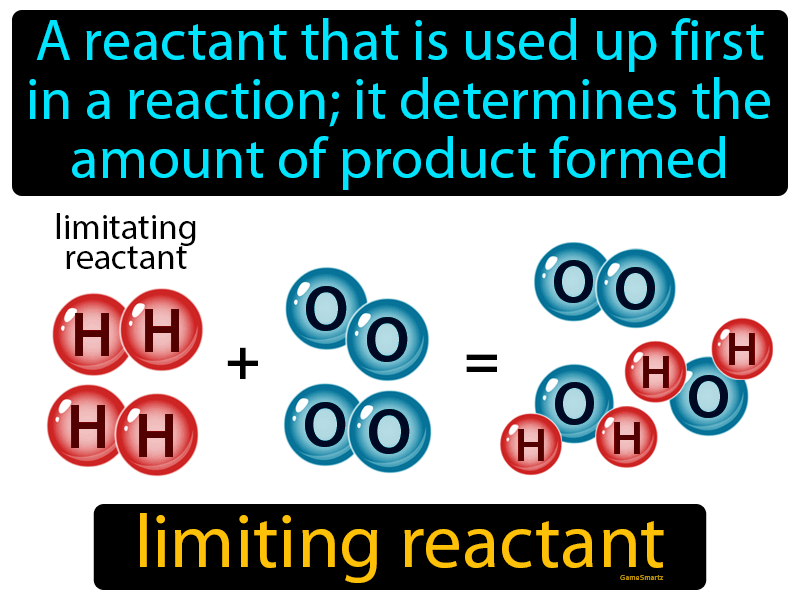
Limiting Reactant Definition Easy To Understand
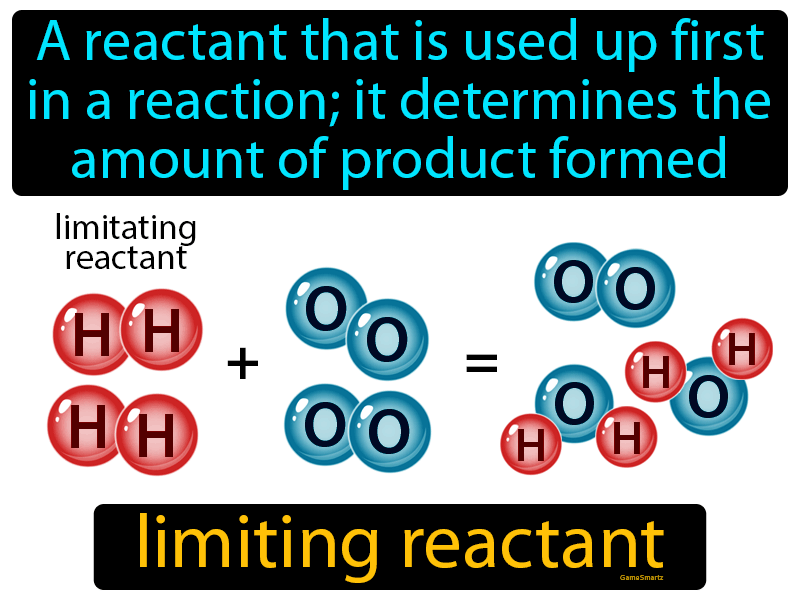
What is a limiting reactant A limiting reactant is the substance that is completely used up when a reaction takes place It is known as a limiting reactant because it limits the reaction so that only a certain maximum amount
The limiting reactant is the reactant that limits a chemical reaction or determines the amount of product that it can produce It is based on stoichiometry or the mole ratio between reactants and products
What Is A Limiting Reactant In Stoichiometry have risen to immense popularity due to numerous compelling reasons:
-
Cost-Efficiency: They eliminate the requirement of buying physical copies or costly software.
-
Modifications: This allows you to modify printables to your specific needs such as designing invitations as well as organizing your calendar, or decorating your home.
-
Educational value: These What Is A Limiting Reactant In Stoichiometry can be used by students from all ages, making them a useful resource for educators and parents.
-
It's easy: instant access various designs and templates is time-saving and saves effort.
Where to Find more What Is A Limiting Reactant In Stoichiometry
Stoichiometry Limiting Reagent AP Review YouTube

Stoichiometry Limiting Reagent AP Review YouTube
The limiting reactant or limiting reagent is the first reactant to get used up in a chemical reaction Once the limiting reactant gets used up the reaction has to stop and cannot continue and there is extra of the other reactants left over
If we add one reactant in excess the other reactant in short supply is the limiting reactant it reacts fully and limits the amount of product formed In the neutralisation example
Now that we've piqued your curiosity about What Is A Limiting Reactant In Stoichiometry and other printables, let's discover where you can discover these hidden treasures:
1. Online Repositories
- Websites such as Pinterest, Canva, and Etsy offer an extensive collection with What Is A Limiting Reactant In Stoichiometry for all applications.
- Explore categories such as decoration for your home, education, management, and craft.
2. Educational Platforms
- Educational websites and forums frequently provide free printable worksheets along with flashcards, as well as other learning tools.
- The perfect resource for parents, teachers as well as students searching for supplementary sources.
3. Creative Blogs
- Many bloggers are willing to share their original designs as well as templates for free.
- These blogs cover a broad range of interests, including DIY projects to party planning.
Maximizing What Is A Limiting Reactant In Stoichiometry
Here are some ways in order to maximize the use of printables for free:
1. Home Decor
- Print and frame stunning artwork, quotes, or other seasonal decorations to fill your living spaces.
2. Education
- Use these printable worksheets free of charge to build your knowledge at home for the classroom.
3. Event Planning
- Create invitations, banners, and decorations for special occasions like weddings or birthdays.
4. Organization
- Stay organized with printable calendars as well as to-do lists and meal planners.
Conclusion
What Is A Limiting Reactant In Stoichiometry are an abundance with useful and creative ideas designed to meet a range of needs and preferences. Their accessibility and versatility make them an invaluable addition to every aspect of your life, both professional and personal. Explore the wide world that is What Is A Limiting Reactant In Stoichiometry today, and explore new possibilities!
Frequently Asked Questions (FAQs)
-
Are printables available for download really are they free?
- Yes they are! You can print and download the resources for free.
-
Can I make use of free printables in commercial projects?
- It depends on the specific terms of use. Always review the terms of use for the creator before utilizing printables for commercial projects.
-
Are there any copyright violations with What Is A Limiting Reactant In Stoichiometry?
- Certain printables could be restricted on use. Be sure to check the terms and conditions offered by the author.
-
How can I print printables for free?
- Print them at home using the printer, or go to an in-store print shop to get the highest quality prints.
-
What software do I require to open printables free of charge?
- A majority of printed materials are in the format PDF. This can be opened with free programs like Adobe Reader.
9 2 Stoichiometry Limiting Reagent Science Chemistry ShowMe
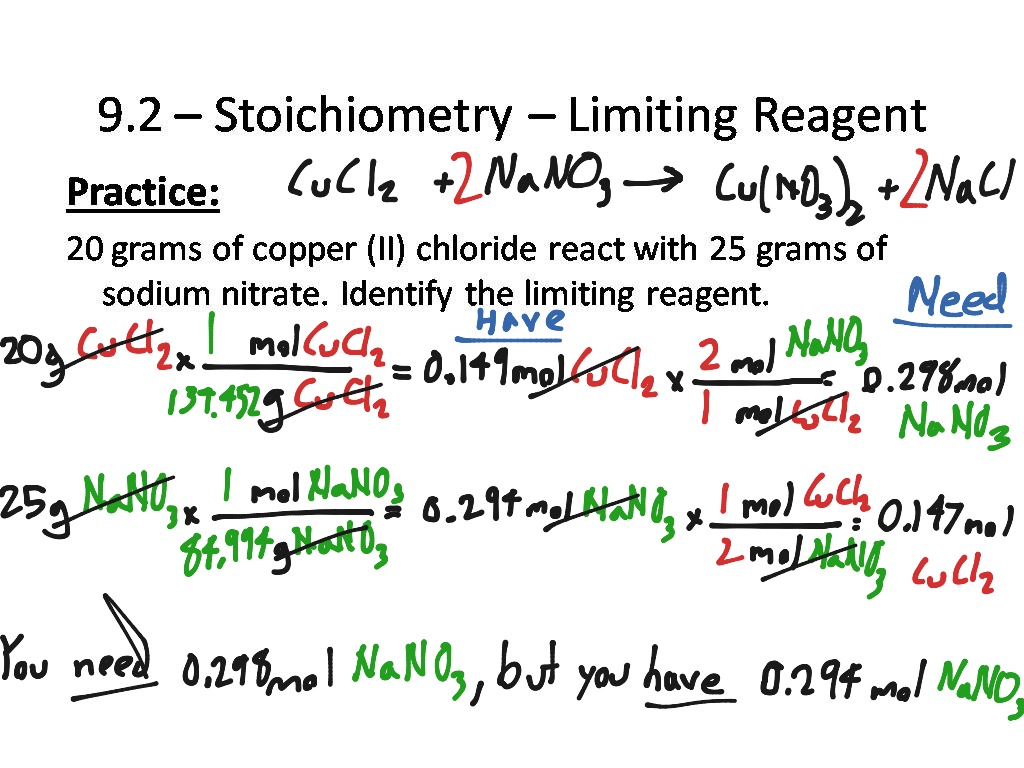
Stoichiometry Limiting Reagent ICE Box YouTube

Check more sample of What Is A Limiting Reactant In Stoichiometry below
Limiting Reactant And Limiting Reagent ChemTalk

STOICHIOMETRY Solving Limiting Reactant Problems In Stoichiometry Easy YouTube
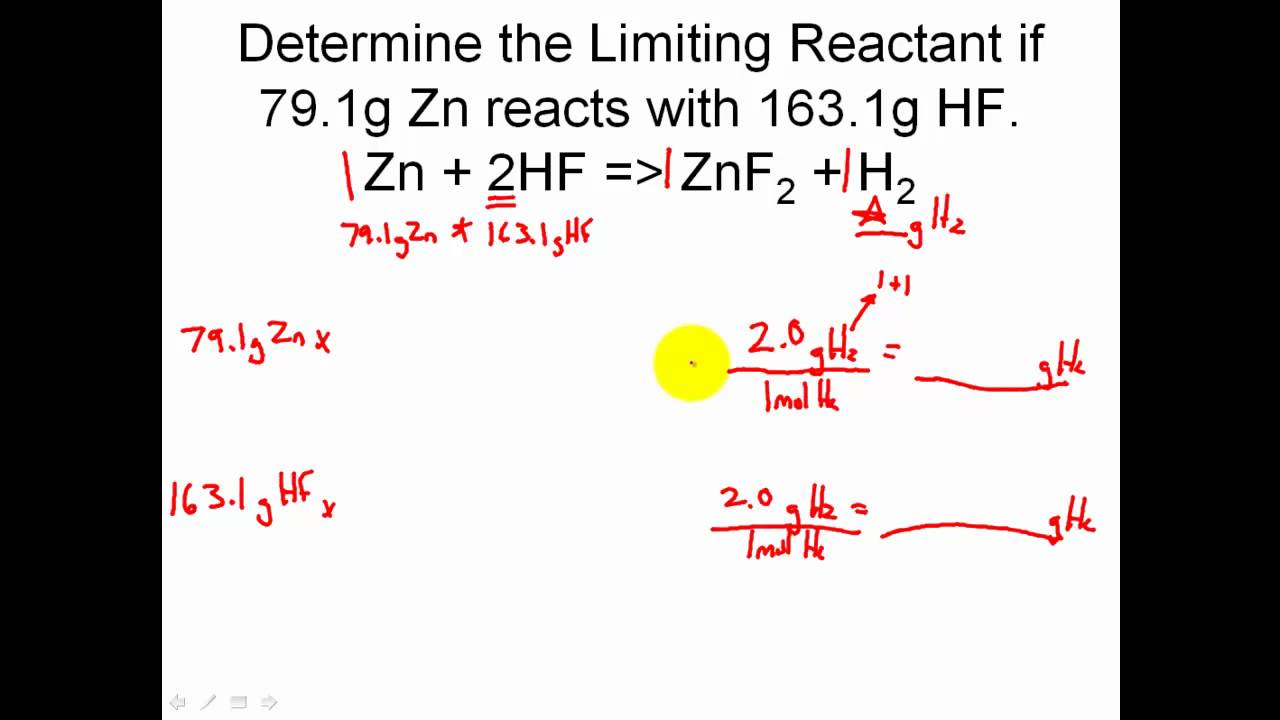
How To Determine Limiting Reactant

Stoichiometry Limiting And Excess Reactant solutions Examples Activities Experiment Videos
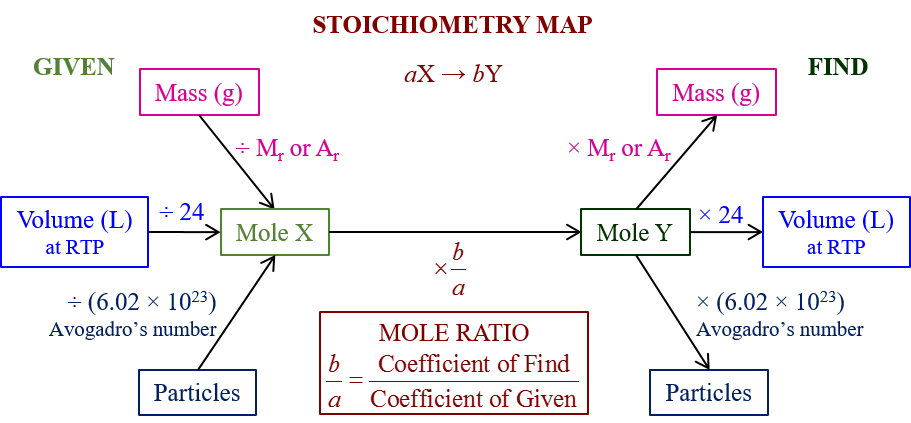
Stoichiometry And Limiting Reactants Limiting Reactant In The Stoichiometry Of Chemical
Limiting Reactant Chemistry Stoichiometry ShowMe
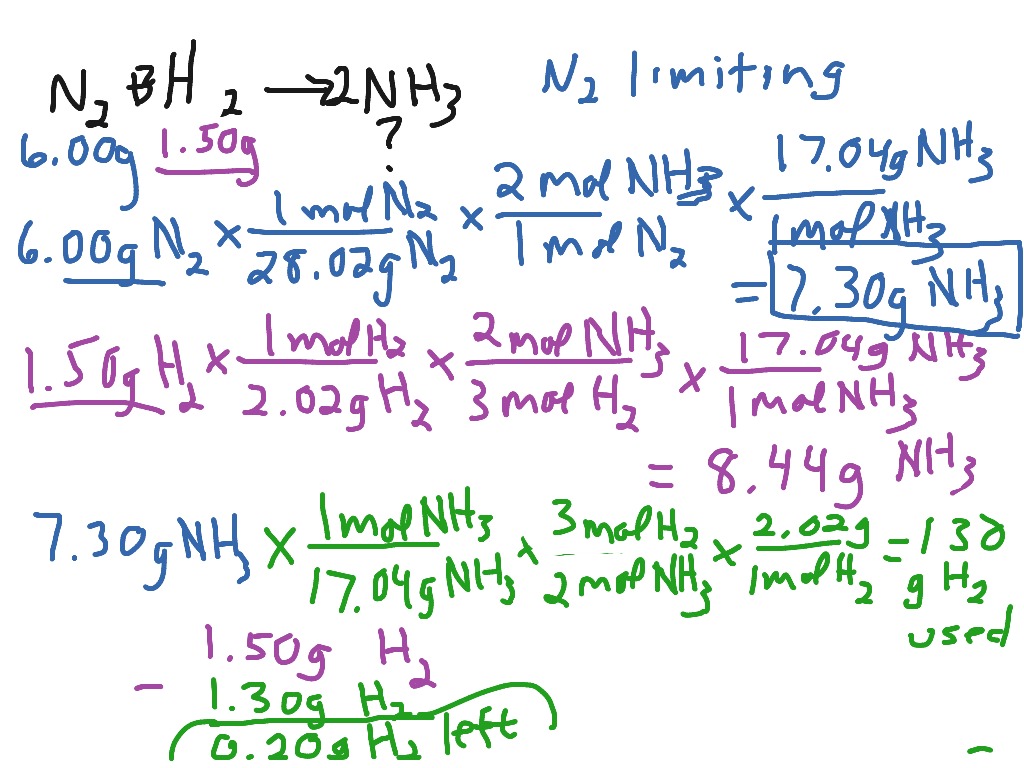
https://chem.libretexts.org › Courses › Anoka-Ramsey...
Identify the limiting reactant limiting reagent in a given chemical reaction Calculate how much product will be produced from the limiting reactant Calculate how much reactant s remains
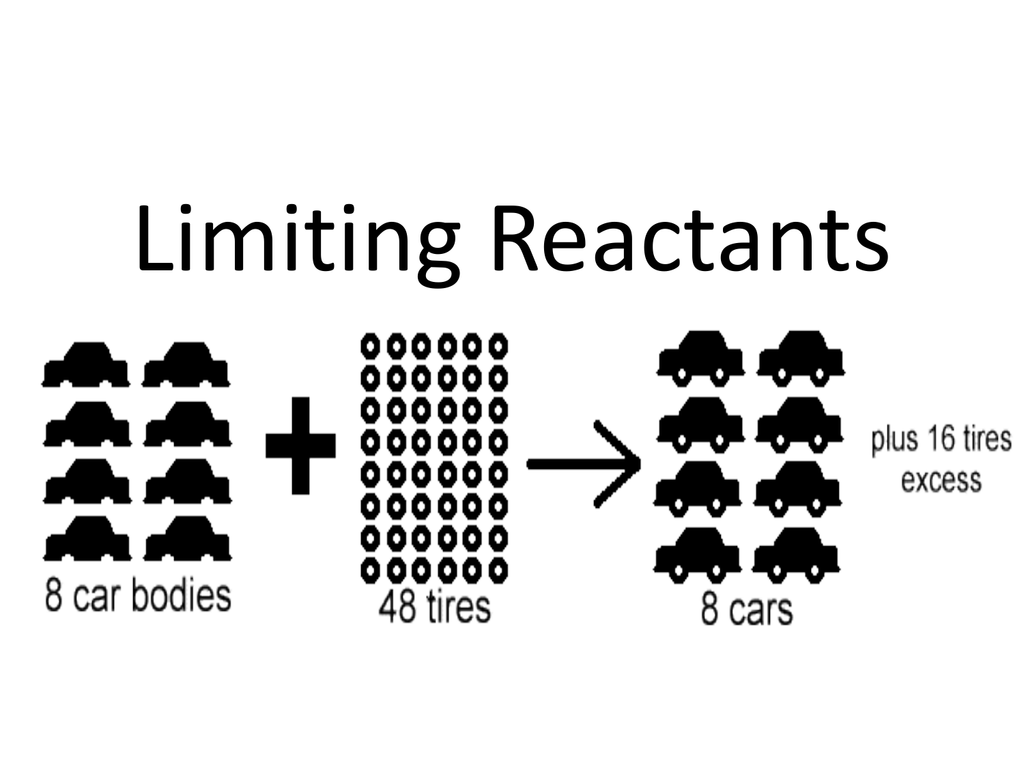
https://general.chemistrysteps.com › limitin…
The limiting reactant is the one that produces less product and not necessarily the one that is present in fewer amounts The correct approach is to calculate the moles of both reactants and using molar conversions determine which one
Identify the limiting reactant limiting reagent in a given chemical reaction Calculate how much product will be produced from the limiting reactant Calculate how much reactant s remains
The limiting reactant is the one that produces less product and not necessarily the one that is present in fewer amounts The correct approach is to calculate the moles of both reactants and using molar conversions determine which one

Stoichiometry Limiting And Excess Reactant solutions Examples Activities Experiment Videos

STOICHIOMETRY Solving Limiting Reactant Problems In Stoichiometry Easy YouTube
.PNG)
Stoichiometry And Limiting Reactants Limiting Reactant In The Stoichiometry Of Chemical
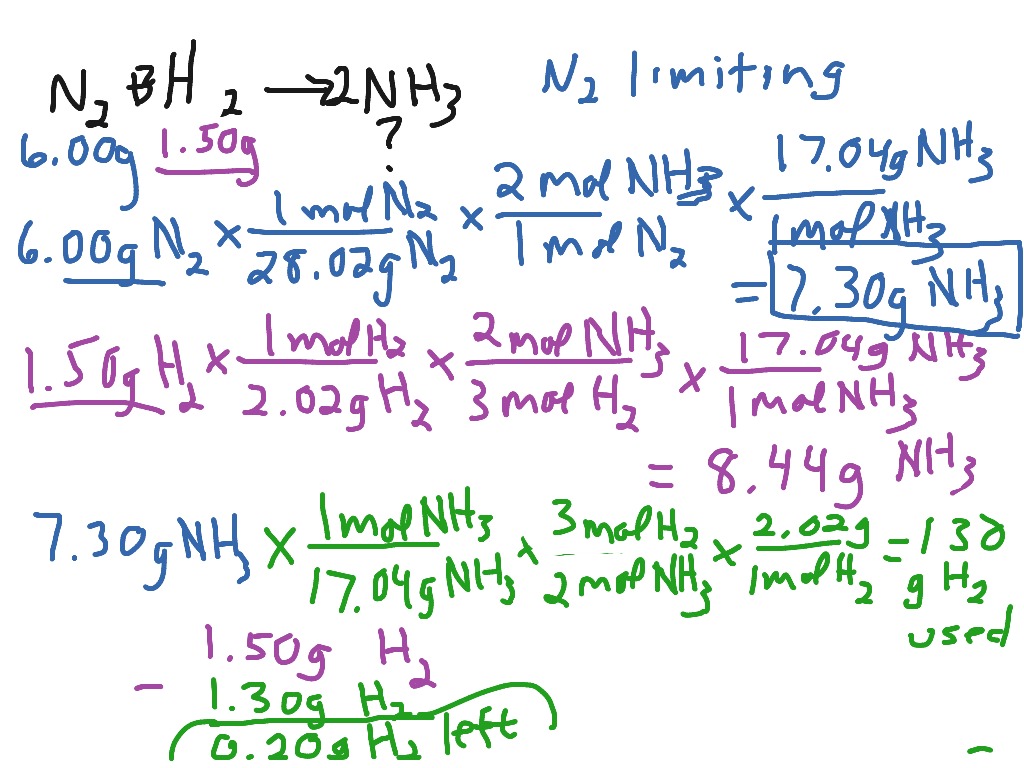
Limiting Reactant Chemistry Stoichiometry ShowMe
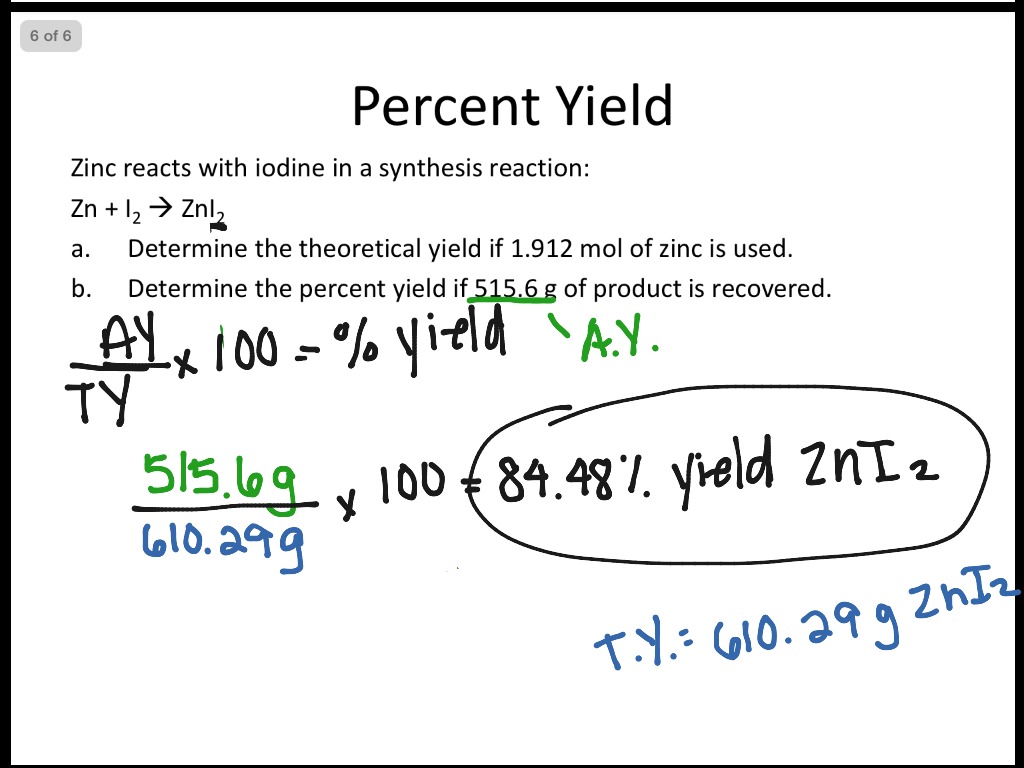
Limiting Reactant And Percent Yield Science Chemistry Stoichiometry ShowMe

STOICHIOMETRY Limiting Reactant Excess Reactant Stoichiometry Moles Module 6 Learning

STOICHIOMETRY Limiting Reactant Excess Reactant Stoichiometry Moles Module 6 Learning
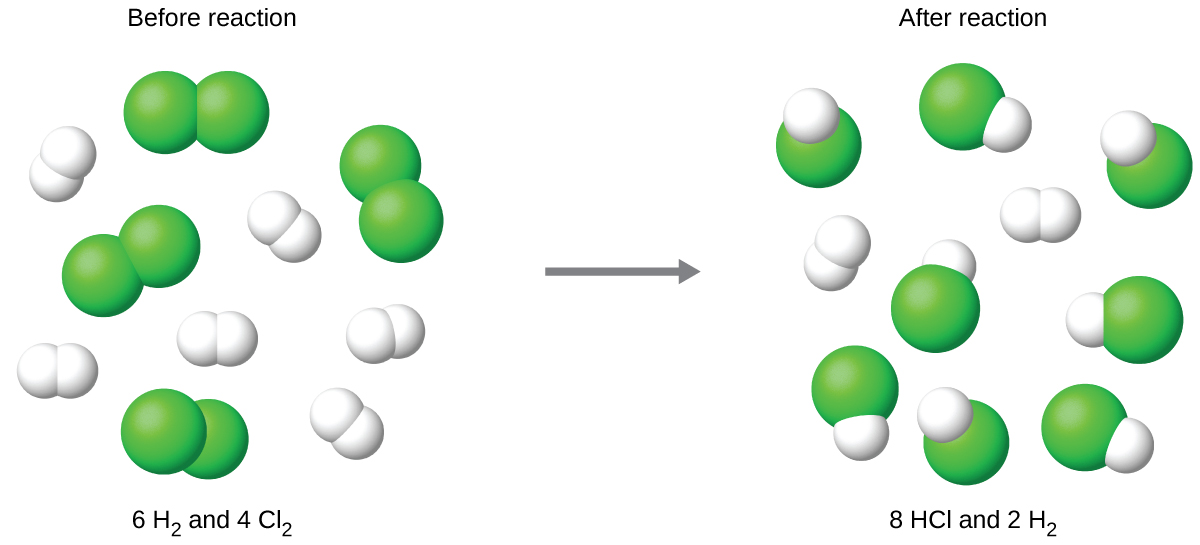
Limiting Reagents Chemistry Activities