In this age of electronic devices, where screens have become the dominant feature of our lives, the charm of tangible printed material hasn't diminished. If it's to aid in education project ideas, artistic or simply to add an individual touch to the area, Steps To Calculate Empirical Formula are now an essential resource. Here, we'll take a dive into the world of "Steps To Calculate Empirical Formula," exploring what they are, where to find them and how they can enhance various aspects of your lives.
Get Latest Steps To Calculate Empirical Formula Below
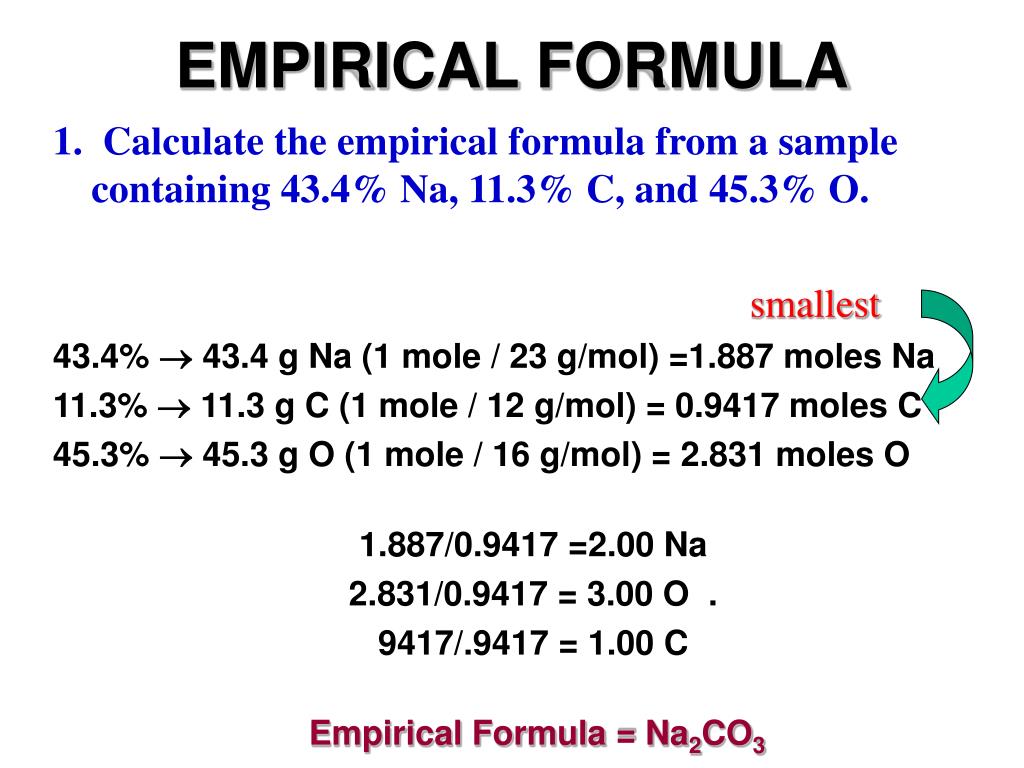
Steps To Calculate Empirical Formula
Steps To Calculate Empirical Formula -
Determining Empirical Formulas An empirical formula is one that shows the lowest whole number ratio of the elements in a compound Because the structure of ionic compounds is an extended three dimensional network of positive and negative ions all formulas of ionic compounds are empirical
To find the empirical formula of a compound start by multiplying the percentage composition of each element by its atomic mass For example if a compound is 40 92 percent carbon multiply 40 92 by 12 its atomic mass to get 3 4
Printables for free include a vast selection of printable and downloadable materials that are accessible online for free cost. These materials come in a variety of types, such as worksheets coloring pages, templates and much more. The attraction of printables that are free lies in their versatility as well as accessibility.
More of Steps To Calculate Empirical Formula
Empirical And Molecular Formula Chemistry Class 11 Some Basic
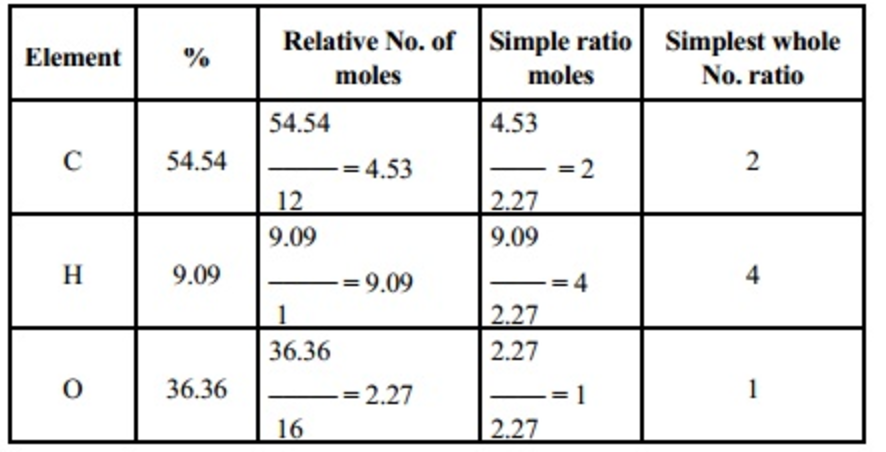
Empirical And Molecular Formula Chemistry Class 11 Some Basic
Basically the mass of the empirical formula can be computed by dividing the molar mass of the compound by it Multiply every atom subscripts by this ratio to compute the molecular formula Solved Examples Problem 1 A compound contains 88 79 oxygen O and 11 19 hydrogen H Compute the empirical formula of the compound Solution
If the molecular or molar mass of the substance is known it may be divided by the empirical formula mass to yield the number of empirical formula units per molecule n molecular or molar mass amu or g mol empirical formula mass amu or g mol n formula units molecule molecular or molar mass amu or g mol empirical formula
Steps To Calculate Empirical Formula have gained a lot of appeal due to many compelling reasons:
-
Cost-Effective: They eliminate the need to purchase physical copies or costly software.
-
Customization: We can customize the design to meet your needs for invitations, whether that's creating them or arranging your schedule or decorating your home.
-
Educational Worth: Printing educational materials for no cost provide for students of all ages, which makes these printables a powerful source for educators and parents.
-
It's easy: The instant accessibility to a myriad of designs as well as templates reduces time and effort.
Where to Find more Steps To Calculate Empirical Formula
What Is Empirical Formula BrockkruwReilly

What Is Empirical Formula BrockkruwReilly
Step 1 Calculate relative mass of the empirical formula Relative empirical mass C x 4 H x 10 S x 1 Relative empirical mass 12 x 4 1 x 10 32 x 1 Relative empirical mass 90 Step 2 Divide relative formula mass of X by relative empirical mass Ratio between M r of X and the M r of the empirical formula 180 90
Steps for Determining an Empirical Formula To calculate the empirical formula we have to first determine the relative masses of the various elements present We may either use mass data in grams or percent composition Also for the percentage composition we may assume the total percent of a compound like 100 and the percentage composition
We've now piqued your interest in printables for free and other printables, let's discover where you can find these treasures:
1. Online Repositories
- Websites such as Pinterest, Canva, and Etsy provide a wide selection of Steps To Calculate Empirical Formula suitable for many needs.
- Explore categories such as the home, decor, craft, and organization.
2. Educational Platforms
- Educational websites and forums usually provide worksheets that can be printed for free for flashcards, lessons, and worksheets. tools.
- It is ideal for teachers, parents, and students seeking supplemental resources.
3. Creative Blogs
- Many bloggers share their creative designs or templates for download.
- The blogs are a vast array of topics, ranging from DIY projects to party planning.
Maximizing Steps To Calculate Empirical Formula
Here are some ideas that you can make use use of Steps To Calculate Empirical Formula:
1. Home Decor
- Print and frame gorgeous art, quotes, or decorations for the holidays to beautify your living spaces.
2. Education
- Print free worksheets to aid in learning at your home or in the classroom.
3. Event Planning
- Design invitations and banners and other decorations for special occasions such as weddings and birthdays.
4. Organization
- Be organized by using printable calendars or to-do lists. meal planners.
Conclusion
Steps To Calculate Empirical Formula are a treasure trove of creative and practical resources catering to different needs and interests. Their access and versatility makes these printables a useful addition to both professional and personal life. Explore the vast collection of Steps To Calculate Empirical Formula and unlock new possibilities!
Frequently Asked Questions (FAQs)
-
Are printables actually available for download?
- Yes, they are! You can print and download these items for free.
-
Can I download free printouts for commercial usage?
- It's determined by the specific conditions of use. Always review the terms of use for the creator prior to printing printables for commercial projects.
-
Are there any copyright issues with Steps To Calculate Empirical Formula?
- Certain printables may be subject to restrictions in use. Be sure to check the terms of service and conditions provided by the creator.
-
How do I print Steps To Calculate Empirical Formula?
- You can print them at home using either a printer at home or in any local print store for the highest quality prints.
-
What software do I require to open printables at no cost?
- A majority of printed materials are with PDF formats, which can be opened with free software like Adobe Reader.
How To Find The Empirical Formula 11 Steps with Pictures

How To Find The Empirical Formula Of A Compound YouTube
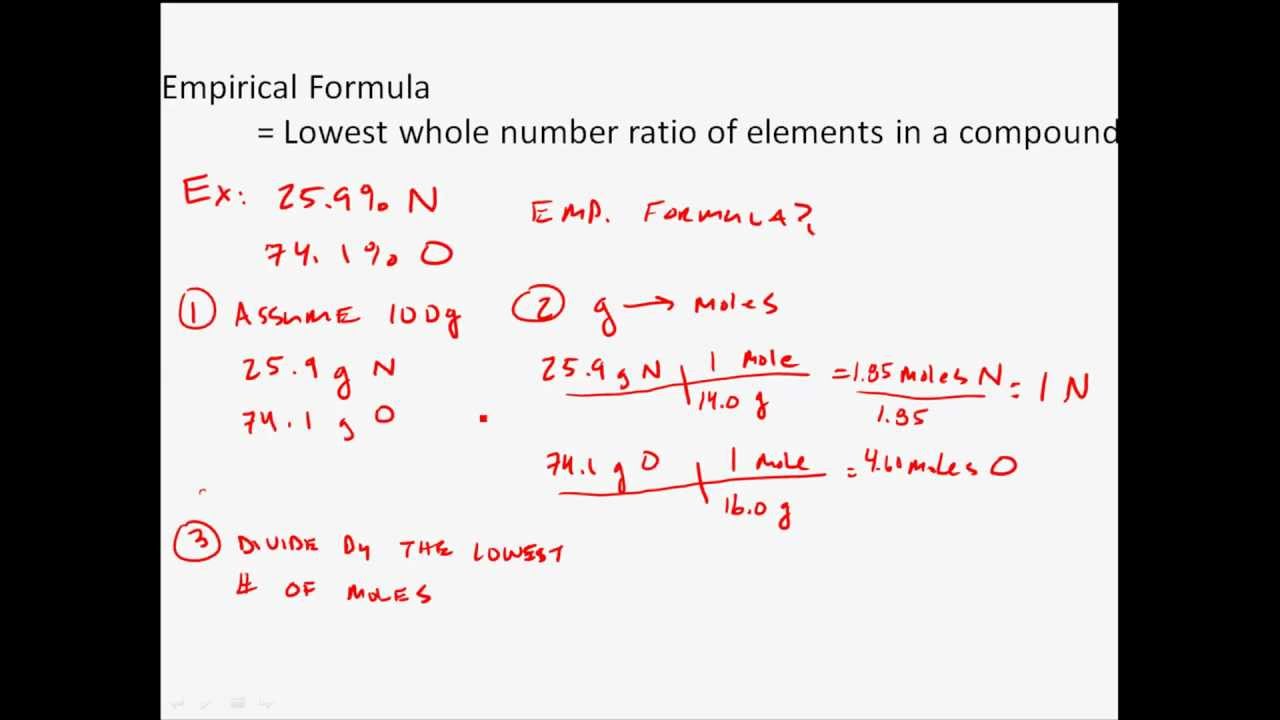
Check more sample of Steps To Calculate Empirical Formula below
How To Determine Empirical Formulas YouTube

PPT EMPIRICAL FORMULA PowerPoint Presentation Free Download ID 6857458
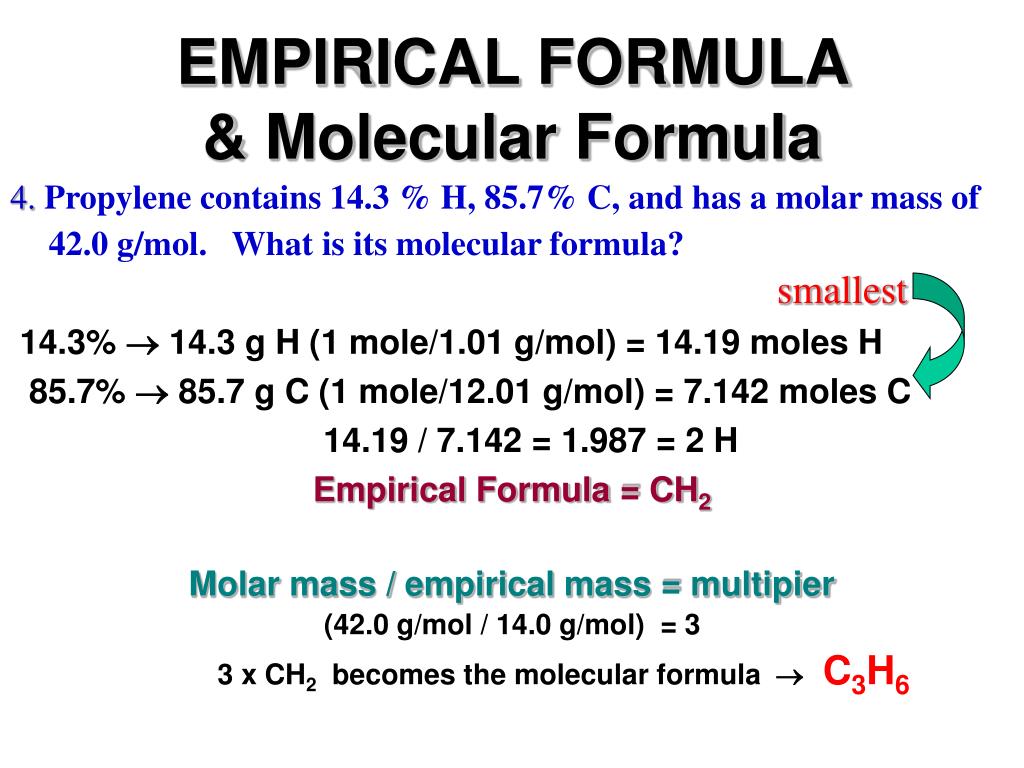
Empirical Formula Definition Get Education
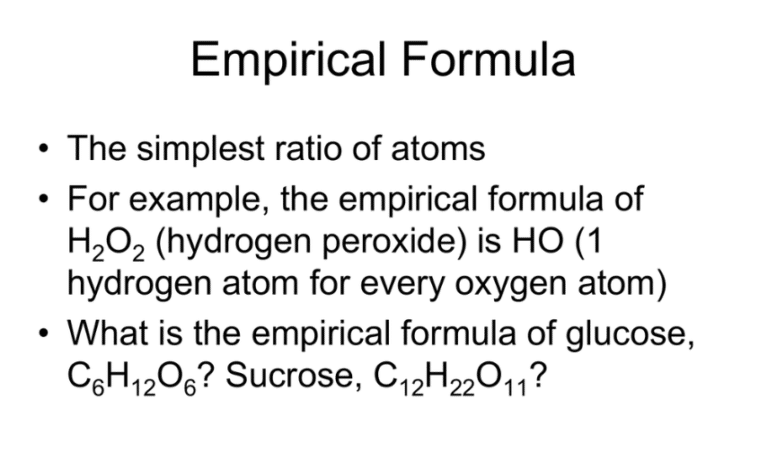
Lesson 4 Empirical Formula YouTube

Empirical Formula
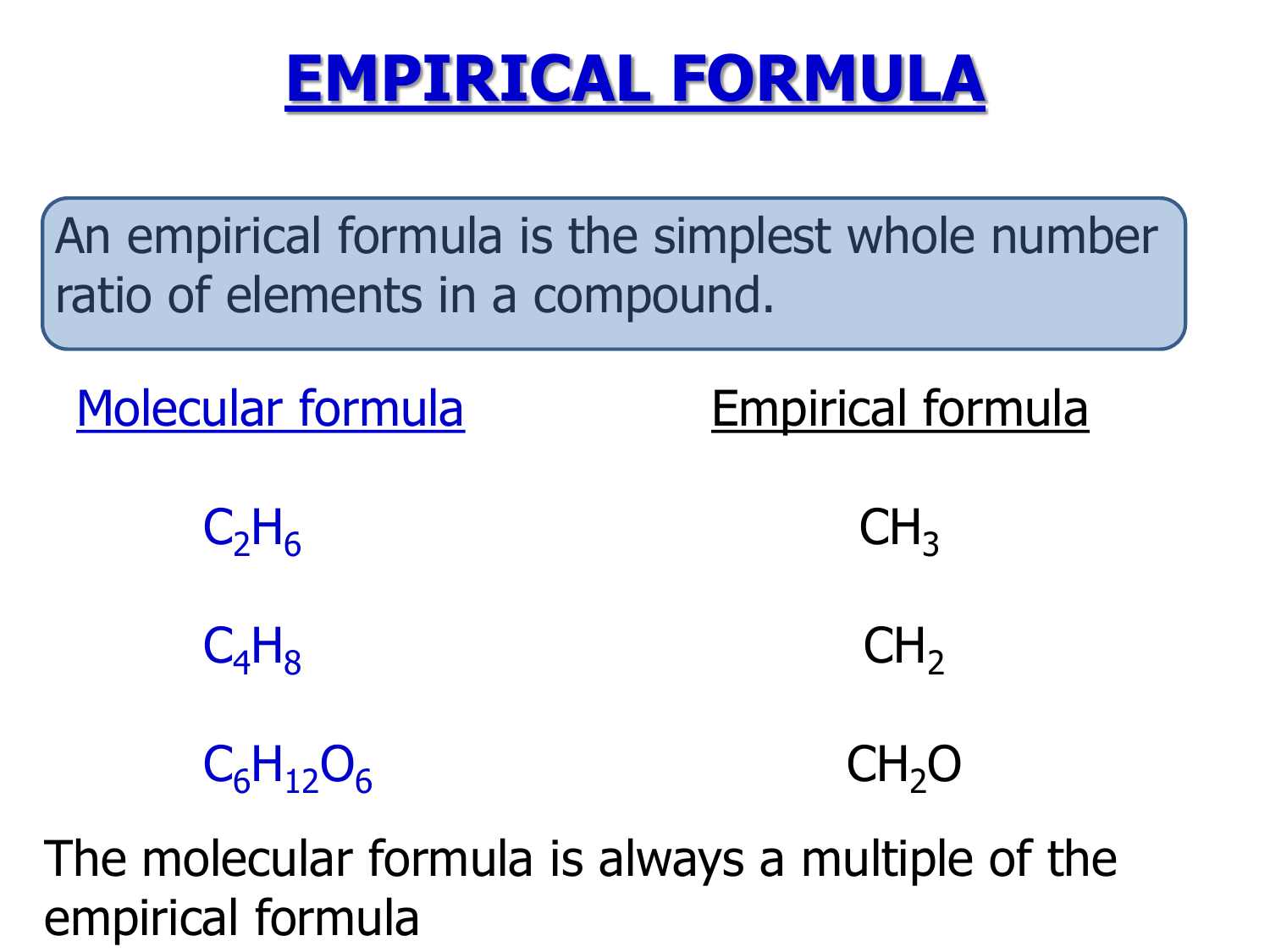
ChemT3am Weekly Reflections Week 23 Empirical Formulas And Applying


https://www.wikihow.com/Find-the-Empirical-Formula
To find the empirical formula of a compound start by multiplying the percentage composition of each element by its atomic mass For example if a compound is 40 92 percent carbon multiply 40 92 by 12 its atomic mass to get 3 4
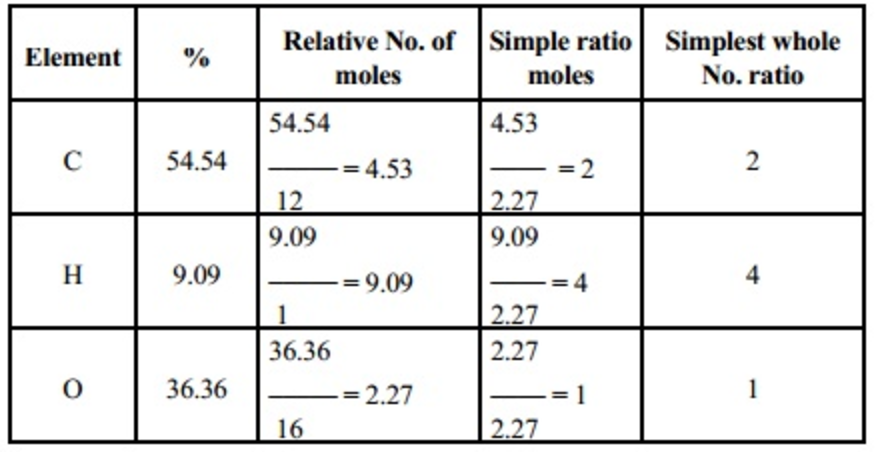
https://www.bbc.co.uk/bitesize/guides/z2ty97h/revision/2
Edexcel Chemistry calculations Edexcel Empirical formulae An empirical formula of a substance is found using the masses and relative atomic masses of the elements it contains The law of
To find the empirical formula of a compound start by multiplying the percentage composition of each element by its atomic mass For example if a compound is 40 92 percent carbon multiply 40 92 by 12 its atomic mass to get 3 4
Edexcel Chemistry calculations Edexcel Empirical formulae An empirical formula of a substance is found using the masses and relative atomic masses of the elements it contains The law of

Lesson 4 Empirical Formula YouTube

PPT EMPIRICAL FORMULA PowerPoint Presentation Free Download ID 6857458

Empirical Formula

ChemT3am Weekly Reflections Week 23 Empirical Formulas And Applying
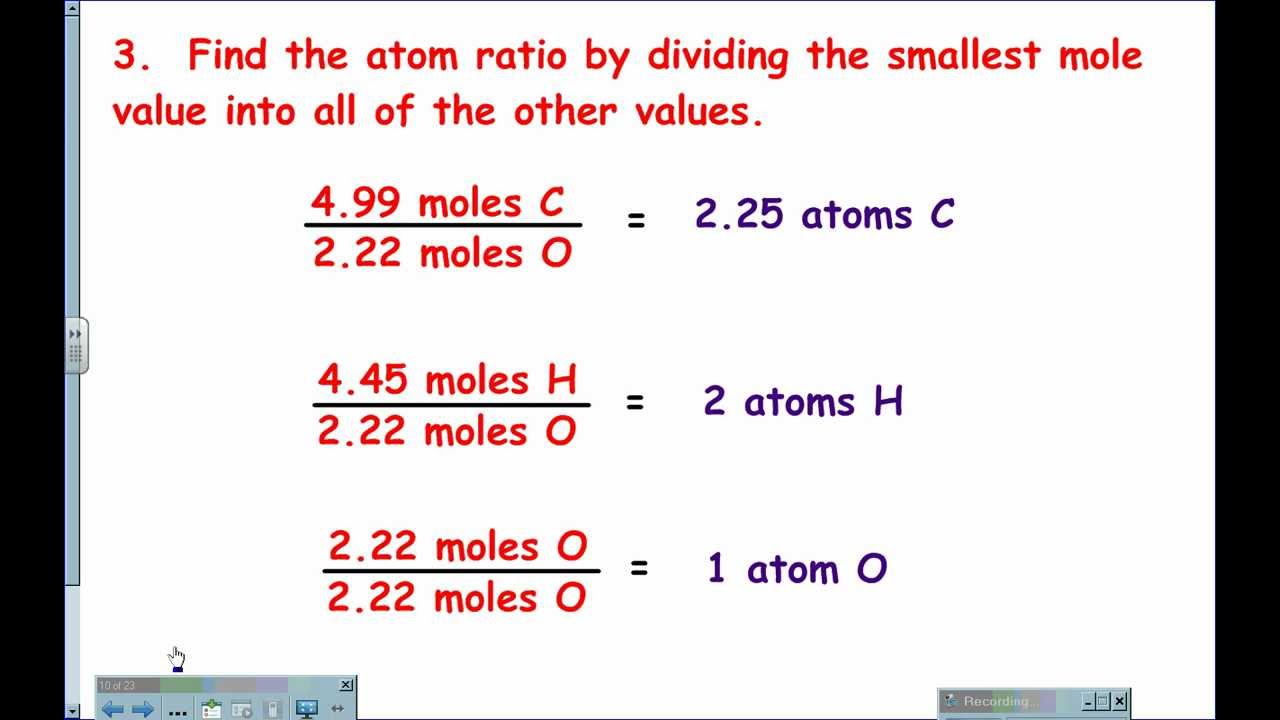
Solving For Empirical Formulas YouTube

Empirical Formula YouTube

Empirical Formula YouTube

A Simple Guide To The Empirical Formula Part 2 YouTube