In the digital age, in which screens are the norm it's no wonder that the appeal of tangible printed objects hasn't waned. If it's to aid in education or creative projects, or simply adding an individual touch to your home, printables for free have proven to be a valuable source. For this piece, we'll take a dive to the depths of "Static Equilibrium In Chemistry," exploring the different types of printables, where they are available, and what they can do to improve different aspects of your life.
Get Latest Static Equilibrium In Chemistry Below

Static Equilibrium In Chemistry
Static Equilibrium In Chemistry -
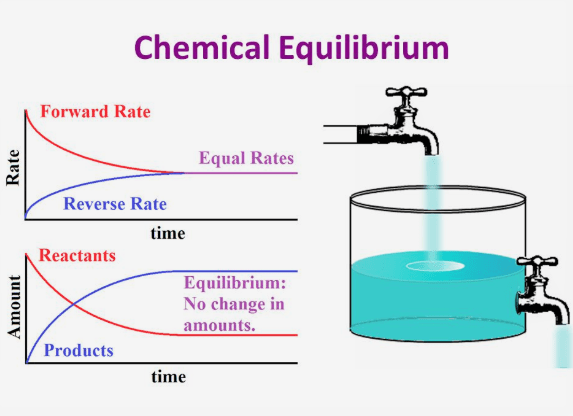
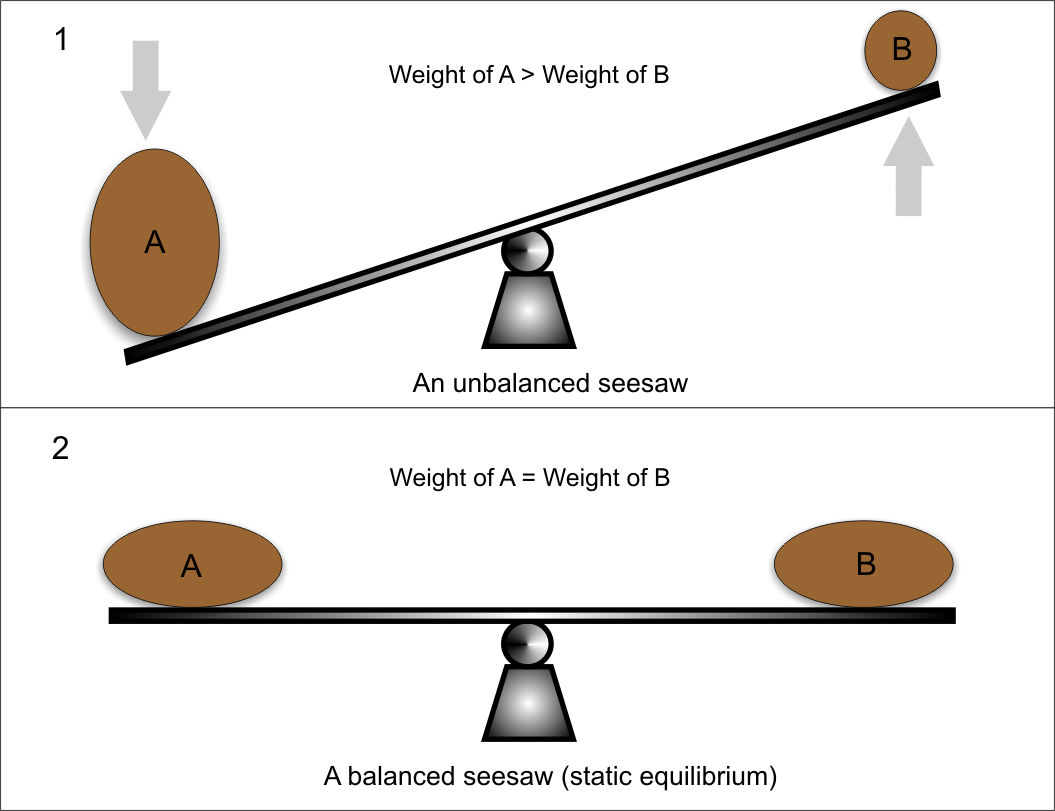
A static equilibrium is a state reached when a reaction goes to completion Figure 1 At this stage the rates of forward and reverse reaction both equal zero Figure 1 diagram illustrates an example of static equilibrium All reactants blue are converted into products yellow The forward and reverse reaction rates are both zero
Static equilibrium is a type of equilibrium in which the rates of the forward and reverse processes are zero We are all familiar with dynamic equilibrium in which the rates of opposing processes are equal Static equilibrium occurs when there is no exchange between reactants and products An example of static equilibrium is
Static Equilibrium In Chemistry provide a diverse assortment of printable, downloadable materials online, at no cost. They are available in a variety of kinds, including worksheets templates, coloring pages and more. The value of Static Equilibrium In Chemistry is their flexibility and accessibility.
More of Static Equilibrium In Chemistry
Dynamic Equilibrium Chemistry LibreTexts

Dynamic Equilibrium Chemistry LibreTexts
Main Difference Static vs Dynamic Equilibrium In chemistry equilibrium refers to a state of a chemical reaction where further changes in the composition of the reactant and the product mixture cannot be perceived from an external point of view However analyzing what happens inside the mixture would give us an idea
Unit 10 Chemical equilibrium This unit is part of the Chemistry library Browse videos articles and exercises by topic
Static Equilibrium In Chemistry have gained immense popularity because of a number of compelling causes:
-
Cost-Efficiency: They eliminate the requirement to purchase physical copies or expensive software.
-
Flexible: Your HTML0 customization options allow you to customize the templates to meet your individual needs whether you're designing invitations to organize your schedule or decorating your home.
-
Educational Value: The free educational worksheets cater to learners of all ages, making them a vital tool for teachers and parents.
-
Simple: immediate access an array of designs and templates saves time and effort.
Where to Find more Static Equilibrium In Chemistry
Chemical Equilibrum Passnownow

Chemical Equilibrum Passnownow
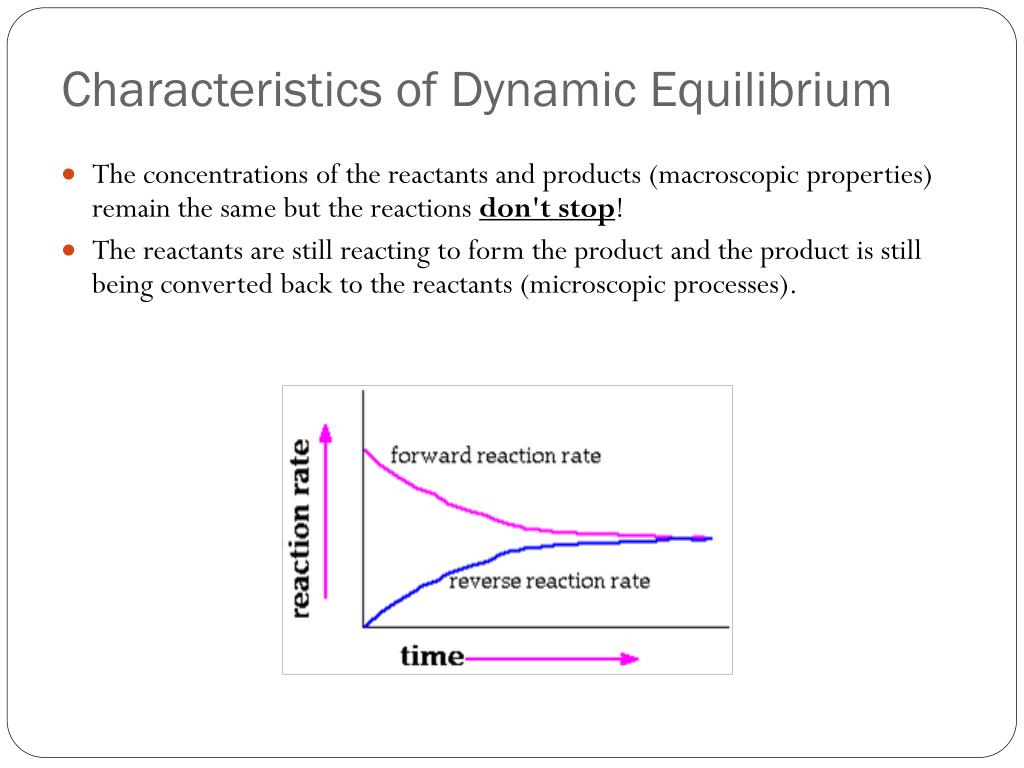
begingroup Since during static equilibrium neither macroscopic nor microscopic particle are change and in dynamic equilibrium the macroscopic particles don t change but the microscopic do so a device which can measure properties at a microscopic level can detect if something is in dynamic or static equilibrium For a chemical reaction
In chemistry equilibrium refers to a state where the rate of a forward reaction is equal to the rate of the reverse reaction There are two main types of equilibrium static and dynamic equilibrium Static equilibrium occurs when there is no net movement of reactants or products in a reaction This can occur when a system is closed and there
In the event that we've stirred your curiosity about Static Equilibrium In Chemistry Let's take a look at where you can find these elusive gems:
1. Online Repositories
- Websites like Pinterest, Canva, and Etsy offer an extensive collection in Static Equilibrium In Chemistry for different uses.
- Explore categories like the home, decor, organizing, and crafts.
2. Educational Platforms
- Forums and educational websites often offer free worksheets and worksheets for printing along with flashcards, as well as other learning materials.
- This is a great resource for parents, teachers, and students seeking supplemental sources.
3. Creative Blogs
- Many bloggers provide their inventive designs as well as templates for free.
- The blogs covered cover a wide selection of subjects, everything from DIY projects to party planning.
Maximizing Static Equilibrium In Chemistry
Here are some innovative ways for you to get the best of Static Equilibrium In Chemistry:
1. Home Decor
- Print and frame beautiful images, quotes, or decorations for the holidays to beautify your living spaces.
2. Education
- Print free worksheets for reinforcement of learning at home either in the schoolroom or at home.
3. Event Planning
- Design invitations, banners, and decorations for special events such as weddings or birthdays.
4. Organization
- Make sure you are organized with printable calendars as well as to-do lists and meal planners.
Conclusion
Static Equilibrium In Chemistry are a treasure trove of practical and innovative resources catering to different needs and needs and. Their availability and versatility make they a beneficial addition to both professional and personal life. Explore the wide world of Static Equilibrium In Chemistry now and open up new possibilities!
Frequently Asked Questions (FAQs)
-
Are the printables you get for free available for download?
- Yes you can! You can print and download these items for free.
-
Can I use the free printables for commercial purposes?
- It's based on specific usage guidelines. Always check the creator's guidelines prior to utilizing the templates for commercial projects.
-
Do you have any copyright issues when you download printables that are free?
- Some printables may contain restrictions regarding usage. Make sure you read the terms of service and conditions provided by the author.
-
How do I print Static Equilibrium In Chemistry?
- You can print them at home using either a printer or go to an in-store print shop to get top quality prints.
-
What program do I require to open printables that are free?
- Most PDF-based printables are available in PDF format. They is open with no cost software like Adobe Reader.
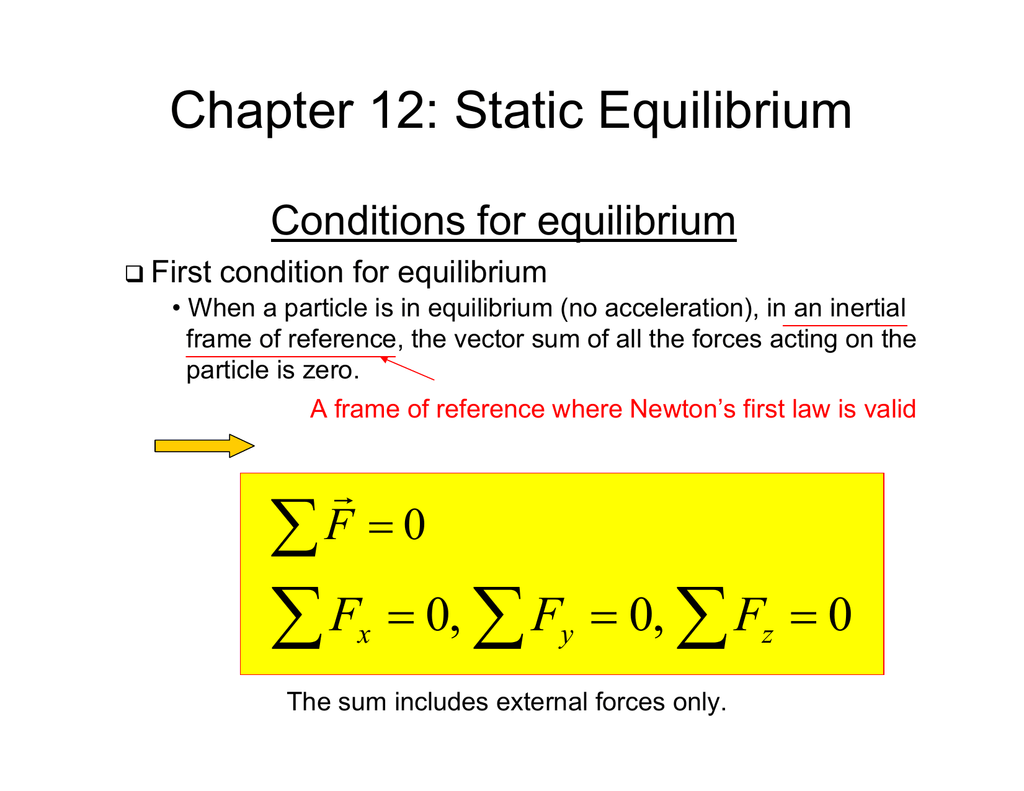
Chapter 12 Static Equilibrium
Dynamic Equilibrium Chemistry LibreTexts

Check more sample of Static Equilibrium In Chemistry below
Chemical Equilibrium ClassNotes ng
Chemical Equilibrium
What Is Meant By Dynamic Equilibrium Brainly in

Chemistry Static Equilibrium Example 2 Money In The

What Is Dynamic Equilibrium Definition And Examples

PPT Equilibrium PowerPoint Presentation Free Download ID 2633301
https://socratic.org/questions/what-is-static-equilibrium-in-chemistry
Static equilibrium is a type of equilibrium in which the rates of the forward and reverse processes are zero We are all familiar with dynamic equilibrium in which the rates of opposing processes are equal Static equilibrium occurs when there is no exchange between reactants and products An example of static equilibrium is

https://chem.libretexts.org/Bookshelves/General...
Figure 13 1 1 A mixture of NO 2 and N 2O 4 moves toward equilibrium Colorless N 2O 4 reacts to form brown NO 2 As the reaction proceeds toward equilibrium the color of the mixture darkens due to the increasing concentration of NO 2 A three part diagram is shown
Static equilibrium is a type of equilibrium in which the rates of the forward and reverse processes are zero We are all familiar with dynamic equilibrium in which the rates of opposing processes are equal Static equilibrium occurs when there is no exchange between reactants and products An example of static equilibrium is
Figure 13 1 1 A mixture of NO 2 and N 2O 4 moves toward equilibrium Colorless N 2O 4 reacts to form brown NO 2 As the reaction proceeds toward equilibrium the color of the mixture darkens due to the increasing concentration of NO 2 A three part diagram is shown

Chemistry Static Equilibrium Example 2 Money In The

Chemical Equilibrium

What Is Dynamic Equilibrium Definition And Examples

PPT Equilibrium PowerPoint Presentation Free Download ID 2633301

7 Important Difference Between Static And Dynamic Equilibrium With
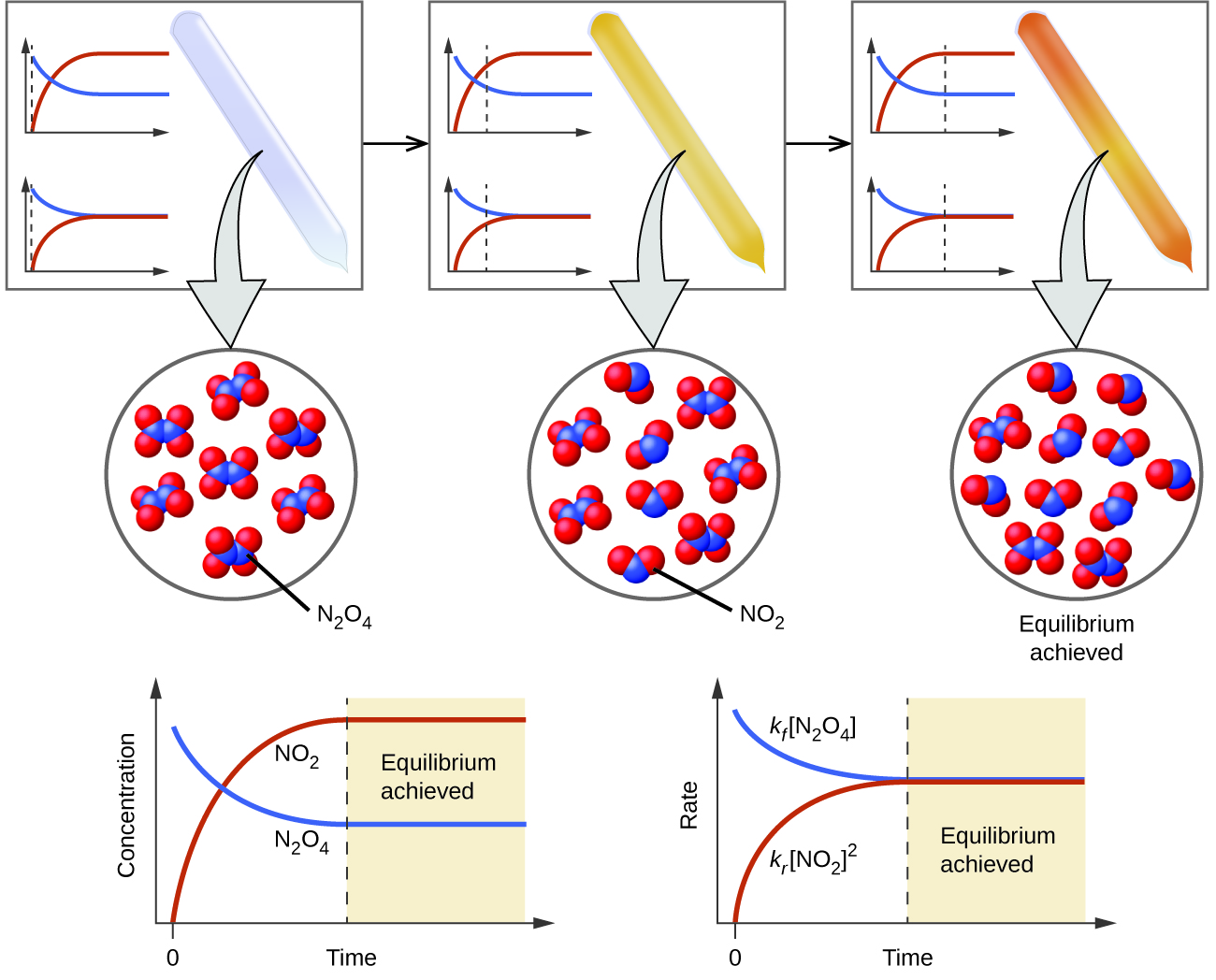
13 1 Chemical Equilibria Chemistry 112 Chapters 12 17 Of OpenStax

13 1 Chemical Equilibria Chemistry 112 Chapters 12 17 Of OpenStax

Chemical Equilibrium At A Glance Teaching Chemistry Chemistry