In the age of digital, where screens rule our lives and the appeal of physical printed objects isn't diminished. For educational purposes and creative work, or simply to add a personal touch to your home, printables for free have become a valuable resource. The following article is a dive in the world of "Rate Law Chemistry," exploring what they are, where you can find them, and ways they can help you improve many aspects of your daily life.
Get Latest Rate Law Chemistry Below
.PNG)
Rate Law Chemistry
Rate Law Chemistry -
The order of a rate law is the sum of the exponents in its concentration terms For the N2O5 decomposition with the rate law k N2O5 this exponent is 1 and thus is not explicitly shown this reaction is therefore a first order reaction We can also say that the reaction is first order in N2O5
Explain the form and function of a rate law Use rate laws to calculate reaction rates Use rate and concentration data to identify reaction orders and derive rate laws As described in the previous module the rate of a reaction is often affected by the concentrations of reactants
Printables for free cover a broad selection of printable and downloadable resources available online for download at no cost. These resources come in many types, like worksheets, templates, coloring pages, and more. The great thing about Rate Law Chemistry lies in their versatility as well as accessibility.
More of Rate Law Chemistry
Integrated Rate Laws Mr Beck s Chemistry
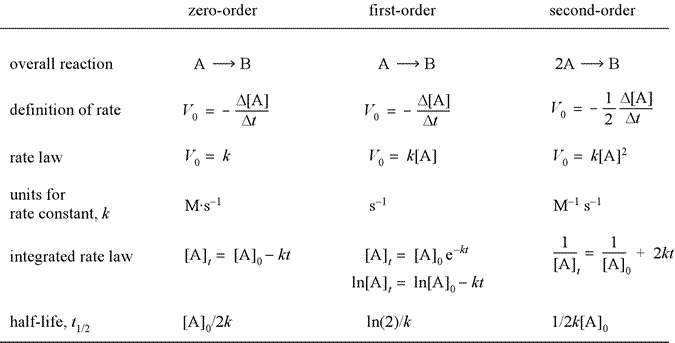
Integrated Rate Laws Mr Beck s Chemistry
Rate laws sometimes called differential rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the concentration of its reactants As an example consider the reaction described by the chemical equation aA bB products a A b B p r o d u c t s
Rate Laws Chemistry for Majors Learning Outcomes Explain the form and function of a rate law Use rate laws to calculate reaction rates Use rate and concentration data to identify reaction orders and derive rate laws As described in the previous module the rate of a reaction is affected by the concentrations of reactants
Rate Law Chemistry have garnered immense popularity due to a myriad of compelling factors:
-
Cost-Effective: They eliminate the need to purchase physical copies or expensive software.
-
Flexible: It is possible to tailor printables to your specific needs whether you're designing invitations to organize your schedule or even decorating your home.
-
Educational Benefits: Downloads of educational content for free provide for students of all ages. This makes them a vital tool for parents and teachers.
-
Easy to use: You have instant access numerous designs and templates saves time and effort.
Where to Find more Rate Law Chemistry
5 7 Using Graphs To Determine Integrated Rate Laws Chemistry LibreTexts
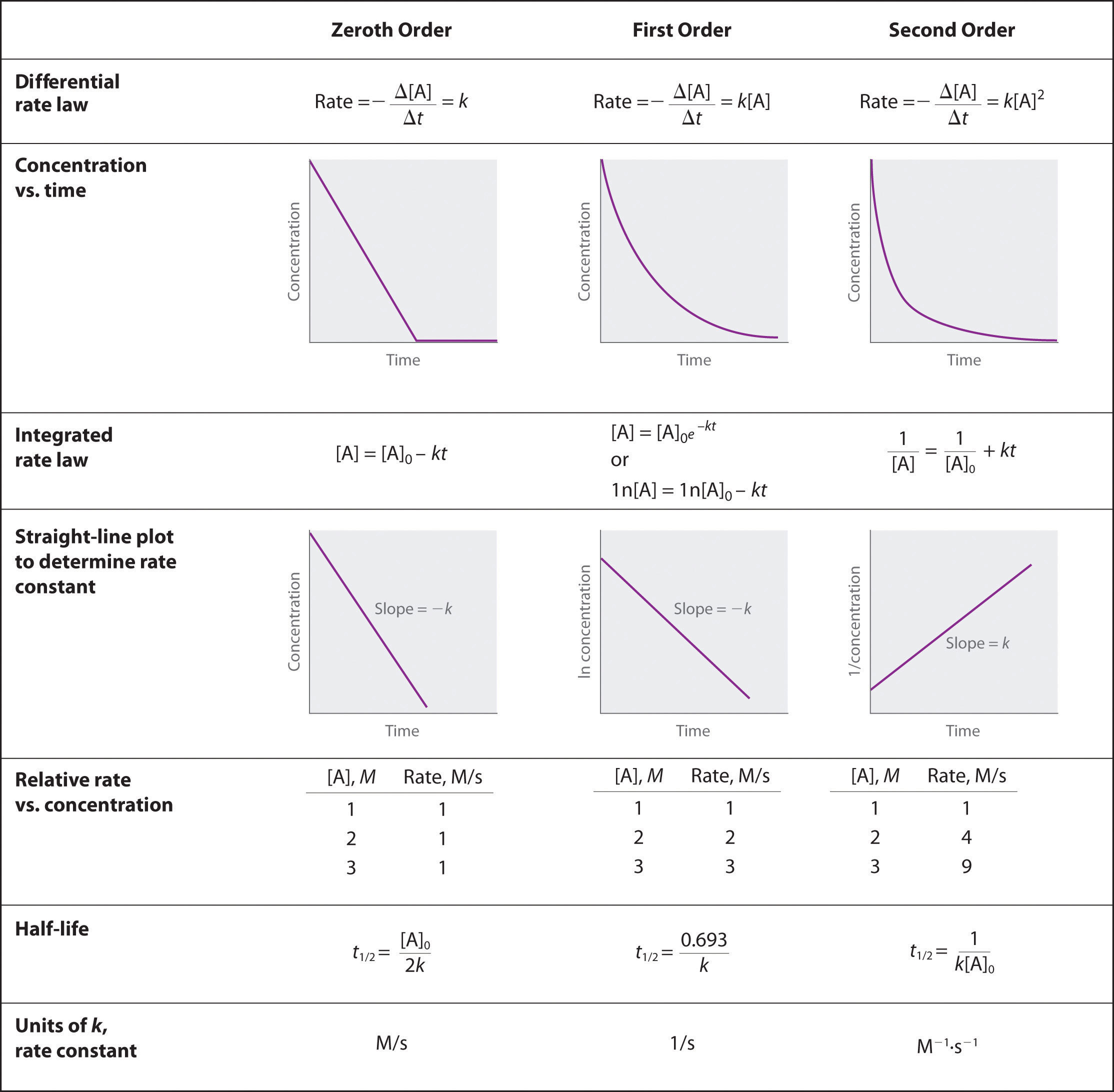
5 7 Using Graphs To Determine Integrated Rate Laws Chemistry LibreTexts
A chemical reaction s rate law is an equation that describes the relationship between the concentrations of reactants in the reaction and the reaction rate In the standard form the rate law equation is written as is reaction rate expressed in concentration unit of time usually molarity second is the specific rate constant
Rate law or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the concentration of its reactants As an example consider the reaction described by the chemical equation aA bB products a A b B products where a and b are stoichiometric coefficients
Since we've got your interest in printables for free Let's look into where the hidden gems:
1. Online Repositories
- Websites such as Pinterest, Canva, and Etsy offer a huge selection of Rate Law Chemistry to suit a variety of applications.
- Explore categories such as interior decor, education, organizational, and arts and crafts.
2. Educational Platforms
- Educational websites and forums often provide free printable worksheets or flashcards as well as learning materials.
- Ideal for parents, teachers as well as students who require additional sources.
3. Creative Blogs
- Many bloggers offer their unique designs and templates at no cost.
- The blogs covered cover a wide range of topics, that includes DIY projects to planning a party.
Maximizing Rate Law Chemistry
Here are some creative ways in order to maximize the use use of printables that are free:
1. Home Decor
- Print and frame stunning artwork, quotes or decorations for the holidays to beautify your living areas.
2. Education
- Use printable worksheets for free to enhance learning at home for the classroom.
3. Event Planning
- Design invitations for banners, invitations and other decorations for special occasions like weddings and birthdays.
4. Organization
- Keep your calendars organized by printing printable calendars checklists for tasks, as well as meal planners.
Conclusion
Rate Law Chemistry are a treasure trove of innovative and useful resources catering to different needs and needs and. Their accessibility and flexibility make they a beneficial addition to each day life. Explore the vast array of Rate Law Chemistry today to discover new possibilities!
Frequently Asked Questions (FAQs)
-
Do printables with no cost really available for download?
- Yes you can! You can print and download these documents for free.
-
Can I utilize free printables to make commercial products?
- It's based on specific terms of use. Make sure you read the guidelines for the creator prior to utilizing the templates for commercial projects.
-
Do you have any copyright issues in printables that are free?
- Certain printables could be restricted in their usage. Always read the terms and condition of use as provided by the author.
-
How can I print Rate Law Chemistry?
- Print them at home using any printer or head to an in-store print shop to get the highest quality prints.
-
What software do I need to open printables for free?
- Most PDF-based printables are available as PDF files, which is open with no cost software such as Adobe Reader.
8 1 Rate Law
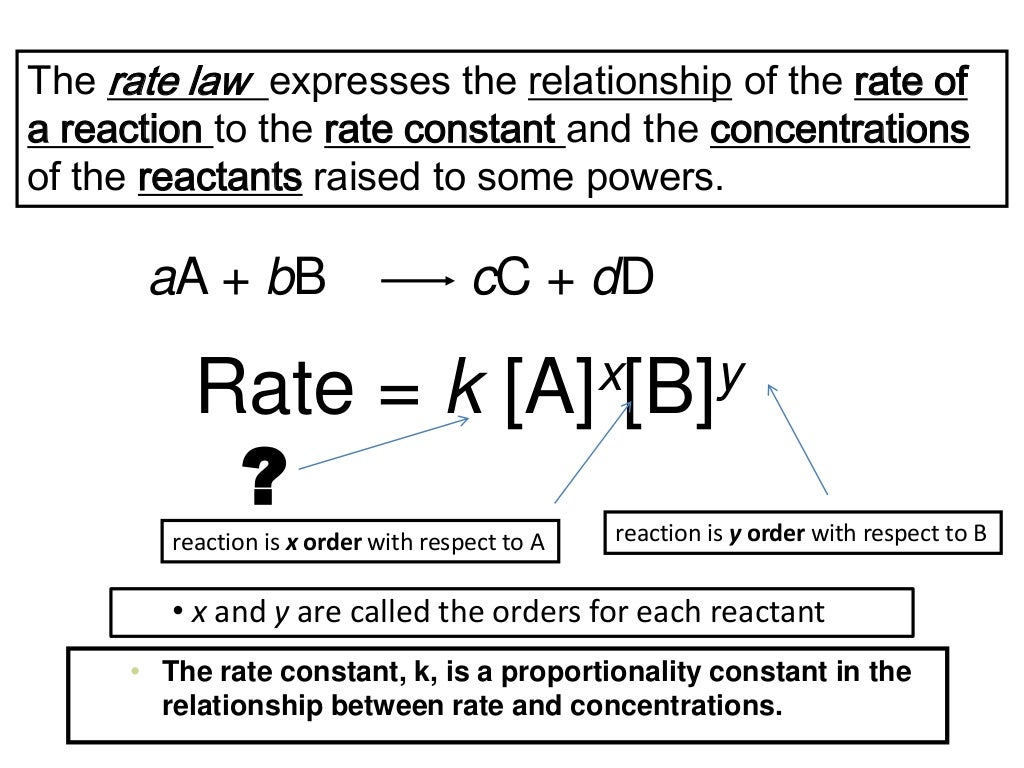
Rate Laws Presentation Chemistry
Check more sample of Rate Law Chemistry below
Rate Law Definition Equation And Examples

Intro To Rate Laws Rate Constants Reaction Order Chemistry Tutorial

Rates Of Reactions Worksheet

Rate Law Https scienceterms chemistry rate law Order Of

Rate Constant Equation Second Order Tessshebaylo
DVHS Chemistry Club Lecture Notes

.PNG?w=186)
https://chem.libretexts.org/Bookshelves/General...
Explain the form and function of a rate law Use rate laws to calculate reaction rates Use rate and concentration data to identify reaction orders and derive rate laws As described in the previous module the rate of a reaction is often affected by the concentrations of reactants

https://byjus.com/chemistry/rate-law-laws-of-motion
The rate law also known as the rate equation for a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it Table of Contents Expression Reaction Orders Rate Constants Differential Rate Equations Integrated Rate Equations
Explain the form and function of a rate law Use rate laws to calculate reaction rates Use rate and concentration data to identify reaction orders and derive rate laws As described in the previous module the rate of a reaction is often affected by the concentrations of reactants
The rate law also known as the rate equation for a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it Table of Contents Expression Reaction Orders Rate Constants Differential Rate Equations Integrated Rate Equations

Rate Law Https scienceterms chemistry rate law Order Of

Intro To Rate Laws Rate Constants Reaction Order Chemistry Tutorial
.PNG)
Rate Constant Equation Second Order Tessshebaylo

DVHS Chemistry Club Lecture Notes
How To Write A Rate Law Expression Given Reaction Order Chemistry

AP Chemistry Kinetics 1 Differential Rate Law Rate Constant YouTube

AP Chemistry Kinetics 1 Differential Rate Law Rate Constant YouTube

Principles Of Chem 2
.PNG)