In this age of electronic devices, with screens dominating our lives yet the appeal of tangible printed materials isn't diminishing. Whether it's for educational purposes and creative work, or simply to add an extra personal touch to your area, Polar Covalent Compounds Dissolve In Water can be an excellent source. Here, we'll dive deeper into "Polar Covalent Compounds Dissolve In Water," exploring what they are, where to find them and the ways that they can benefit different aspects of your lives.
Get Latest Polar Covalent Compounds Dissolve In Water Below

Polar Covalent Compounds Dissolve In Water
Polar Covalent Compounds Dissolve In Water -
To answer the first question a molecule must have a charge to dissolve in water because for a substance to dissolve it has to form bonds with the solvent and it cant do that unless it is polar 2 It happens with polar molecules like methanol as
Polar species are soluble in water while nonpolar species are soluble in oils and fats Covalent solubility uses the like dissolves like rule This means that substances with the same type of polarity will be soluble in one another Moreover compounds with differing polarities will be insoluble in one another
Printables for free cover a broad assortment of printable documents that can be downloaded online at no cost. They are available in a variety of forms, including worksheets, coloring pages, templates and many more. The benefit of Polar Covalent Compounds Dissolve In Water is their flexibility and accessibility.
More of Polar Covalent Compounds Dissolve In Water
PPT Water And It s Properties PowerPoint Presentation ID 6237263

PPT Water And It s Properties PowerPoint Presentation ID 6237263
Water and other polar molecules are attracted to ions as shown in Figure 4 10 2 4 10 2 The electrostatic attraction between an ion and a molecule with a dipole is called an ion dipole attraction These attractions play an important role in the dissolution of ionic compounds in water
The polarity of a covalent bond can be judged by determining the difference of the electronegativities of the two atoms involved in the covalent bond as summarized in the following table Electronegativity Difference
Printables that are free have gained enormous popularity for several compelling reasons:
-
Cost-Effective: They eliminate the requirement to purchase physical copies of the software or expensive hardware.
-
Customization: The Customization feature lets you tailor printables to fit your particular needs such as designing invitations to organize your schedule or decorating your home.
-
Educational value: Printables for education that are free are designed to appeal to students of all ages. This makes these printables a powerful tool for teachers and parents.
-
Convenience: Instant access to a variety of designs and templates cuts down on time and efforts.
Where to Find more Polar Covalent Compounds Dissolve In Water
Polar Molecules Dissolve In Water Canvas depot

Polar Molecules Dissolve In Water Canvas depot
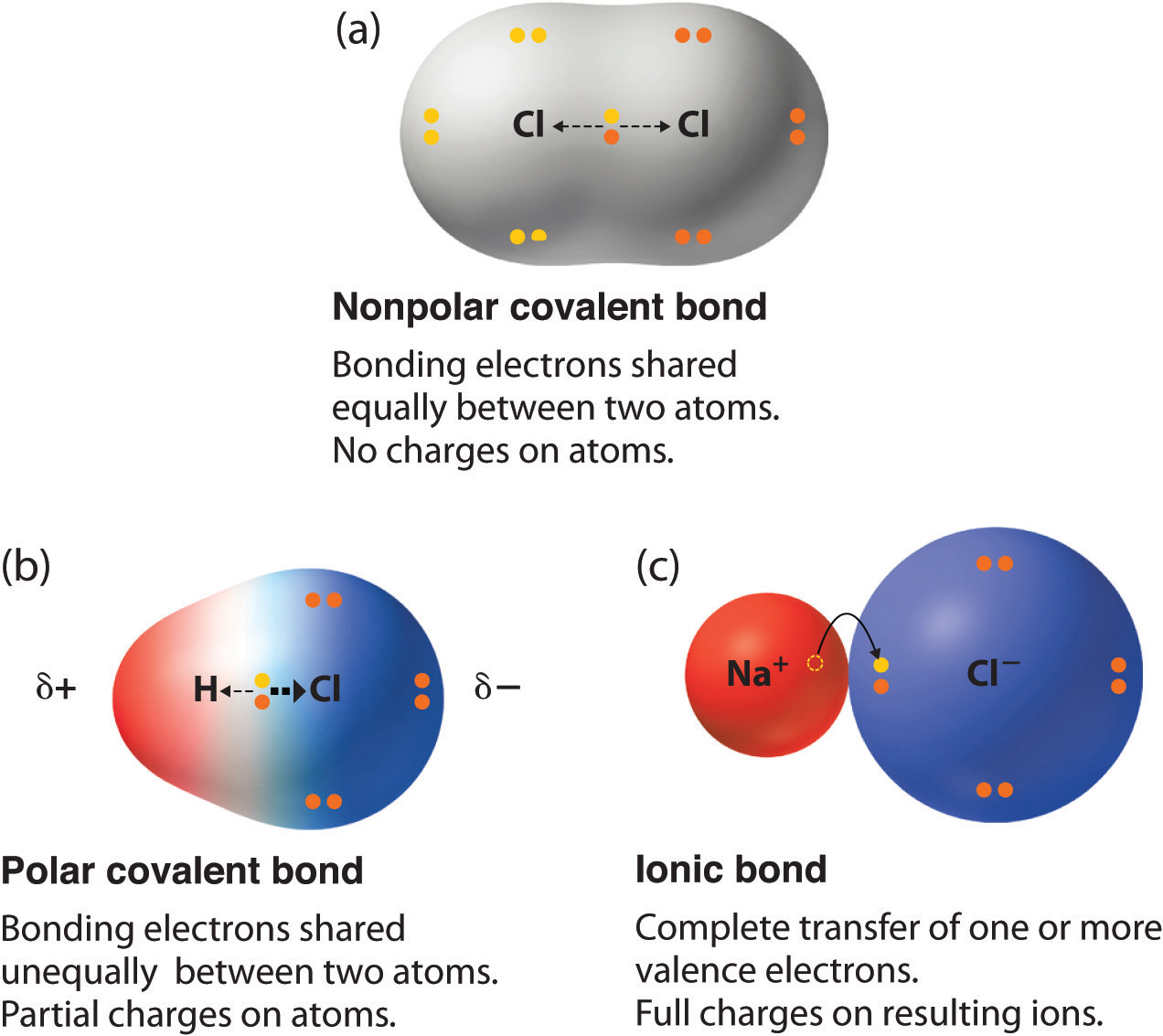
The covalent bond formed between two atoms in molecules whose electronegative difference exists is known as a polar covalent bond Explanation of Polar Covalent Bond Polar covalent bonds are usually formed between two nonmetal atoms having different electronegativities
Ionic solutes that are able to participate in these interactions will dissolve in water If you have a polar compound right a similar idea you have attractive forces that allow the polar compounds to be dissolved in a polar solvent like water
We've now piqued your interest in printables for free Let's see where you can find these elusive gems:
1. Online Repositories
- Websites like Pinterest, Canva, and Etsy provide a wide selection of Polar Covalent Compounds Dissolve In Water suitable for many needs.
- Explore categories like the home, decor, organizational, and arts and crafts.
2. Educational Platforms
- Educational websites and forums often offer free worksheets and worksheets for printing with flashcards and other teaching materials.
- The perfect resource for parents, teachers, and students seeking supplemental sources.
3. Creative Blogs
- Many bloggers share their creative designs and templates at no cost.
- These blogs cover a broad variety of topics, ranging from DIY projects to party planning.
Maximizing Polar Covalent Compounds Dissolve In Water
Here are some unique ways of making the most use of printables that are free:
1. Home Decor
- Print and frame beautiful artwork, quotes or decorations for the holidays to beautify your living areas.
2. Education
- Print out free worksheets and activities to enhance your learning at home and in class.
3. Event Planning
- Design invitations and banners and other decorations for special occasions such as weddings or birthdays.
4. Organization
- Be organized by using printable calendars, to-do lists, and meal planners.
Conclusion
Polar Covalent Compounds Dissolve In Water are an abundance of creative and practical resources that meet a variety of needs and interests. Their accessibility and versatility make them a fantastic addition to the professional and personal lives of both. Explore the plethora that is Polar Covalent Compounds Dissolve In Water today, and discover new possibilities!
Frequently Asked Questions (FAQs)
-
Are printables available for download really cost-free?
- Yes you can! You can print and download these tools for free.
-
Does it allow me to use free printing templates for commercial purposes?
- It depends on the specific terms of use. Always consult the author's guidelines prior to printing printables for commercial projects.
-
Do you have any copyright rights issues with Polar Covalent Compounds Dissolve In Water?
- Some printables may have restrictions on use. Always read the terms and conditions set forth by the designer.
-
How do I print Polar Covalent Compounds Dissolve In Water?
- Print them at home with printing equipment or visit a local print shop for better quality prints.
-
What program do I require to open printables at no cost?
- Most PDF-based printables are available in PDF format, which can be opened using free software such as Adobe Reader.
What Is Produced Through Dissolving Ionic Compounds In Water

Polar Molecules Water s Ability To Dissolve Other Molecules

Check more sample of Polar Covalent Compounds Dissolve In Water below
Definition And Examples Of A Polar Bond
/PolarConvalentBond-58a715be3df78c345b77b57d.jpg)
Ms J s Chemistry Class Polar Vs NonPolar Covalent Bonds
Zolmovies Mass Of Sugar Dissolved In Water
Organic Chemistry If All Intermolecular Forces Are Electrostatic In

Covalent Compounds Examples And Properties

CH150 Chapter 7 Solutions Chemistry


https://chem.libretexts.org/Courses/Furman...
Polar species are soluble in water while nonpolar species are soluble in oils and fats Covalent solubility uses the like dissolves like rule This means that substances with the same type of polarity will be soluble in one another Moreover compounds with differing polarities will be insoluble in one another

https://www.chemistrylearner.com/chemical-bonds/...
Water H 2 O Water is a polar solvent A polar covalent bond is created when the oxygen O atom more electronegative than hydrogen pulls the shared electrons towards itself As a result the oxygen atom has a partial negative charge associated with it
Polar species are soluble in water while nonpolar species are soluble in oils and fats Covalent solubility uses the like dissolves like rule This means that substances with the same type of polarity will be soluble in one another Moreover compounds with differing polarities will be insoluble in one another
Water H 2 O Water is a polar solvent A polar covalent bond is created when the oxygen O atom more electronegative than hydrogen pulls the shared electrons towards itself As a result the oxygen atom has a partial negative charge associated with it

Organic Chemistry If All Intermolecular Forces Are Electrostatic In

Ms J s Chemistry Class Polar Vs NonPolar Covalent Bonds

Covalent Compounds Examples And Properties

CH150 Chapter 7 Solutions Chemistry

Chemical Bonds Properties Of Ionic And Covalent Compounds

Nonpolar Covalent Bond Definition And Examples

Nonpolar Covalent Bond Definition And Examples

Why Is Water A Polar Molecule In 2020 Covalent Bonding Molecular
