In this day and age where screens have become the dominant feature of our lives it's no wonder that the appeal of tangible printed objects isn't diminished. No matter whether it's for educational uses project ideas, artistic or simply adding the personal touch to your area, Limiting Reagent Questions can be an excellent source. The following article is a take a dive to the depths of "Limiting Reagent Questions," exploring their purpose, where they are available, and what they can do to improve different aspects of your life.
Get Latest Limiting Reagent Questions Below

Limiting Reagent Questions
Limiting Reagent Questions -
A limiting reagent or limiting reactant is a substance that has been wholly consumed in a chemical reaction Thus the limiting reagent determines when to complete and stop a reaction Since the limiting reagent is consumed in a reaction no amount remains to react with another reactant
Which is the limiting reagent Solution path 1 1 Calculate moles of sucrose 10 0 g 342 2948 g mol 0 0292146 mol 2 Calculate moles of oxygen required to react with moles of sucrose From the coefficients we see that 12 moles of oxygen are require for every one mole of sucrose Therefore
Printables for free include a vast assortment of printable, downloadable materials that are accessible online for free cost. These resources come in many forms, like worksheets coloring pages, templates and many more. The appeal of printables for free is their flexibility and accessibility.
More of Limiting Reagent Questions
Percent Yield Practice Worksheet

Percent Yield Practice Worksheet
If you define limiting reagent it is a reactant in a chemical reaction which determines the amount of product which is produced The reason for using a limiting reactant is that the elements and compounds react with each other in a balanced chemical equation according to the mole ratio between them
Learn Limiting Reagent with free step by step video explanations and practice problems by experienced tutors
Limiting Reagent Questions have gained a lot of popularity due to a variety of compelling reasons:
-
Cost-Efficiency: They eliminate the need to buy physical copies of the software or expensive hardware.
-
Flexible: There is the possibility of tailoring the templates to meet your individual needs such as designing invitations to organize your schedule or even decorating your house.
-
Educational Impact: These Limiting Reagent Questions cater to learners from all ages, making them a valuable tool for parents and teachers.
-
Convenience: instant access a variety of designs and templates saves time and effort.
Where to Find more Limiting Reagent Questions
LIMITING REAGENT NUMERICAL SOLUTION 2066 Q No 23 NEB PAST

LIMITING REAGENT NUMERICAL SOLUTION 2066 Q No 23 NEB PAST
The reagent that we run out of first is called the limiting reagent because it limits how long the reaction will go on We say that the other reagent s are in excess There will be some of these reagents left over when the reaction has run its course To find the limiting reagent we must compare the molar amounts of reagents to the
The test tubes are limiting they ran out first and the stoppers are in excess we have some left over when the limiting reagent ran out There are two techniques for determine the limiting reagent in chemical problems The first technique is discussed as part of the solution to the first example
Now that we've piqued your interest in printables for free, let's explore where the hidden gems:
1. Online Repositories
- Websites such as Pinterest, Canva, and Etsy offer a huge selection and Limiting Reagent Questions for a variety motives.
- Explore categories like decorating your home, education, organizing, and crafts.
2. Educational Platforms
- Educational websites and forums often provide free printable worksheets or flashcards as well as learning materials.
- Perfect for teachers, parents and students in need of additional resources.
3. Creative Blogs
- Many bloggers provide their inventive designs with templates and designs for free.
- The blogs covered cover a wide range of interests, everything from DIY projects to planning a party.
Maximizing Limiting Reagent Questions
Here are some creative ways in order to maximize the use of printables for free:
1. Home Decor
- Print and frame gorgeous artwork, quotes or decorations for the holidays to beautify your living spaces.
2. Education
- Use free printable worksheets to enhance your learning at home as well as in the class.
3. Event Planning
- Create invitations, banners, and decorations for special events like weddings or birthdays.
4. Organization
- Be organized by using printable calendars along with lists of tasks, and meal planners.
Conclusion
Limiting Reagent Questions are a treasure trove of innovative and useful resources that can meet the needs of a variety of people and interests. Their accessibility and flexibility make them a great addition to the professional and personal lives of both. Explore the world that is Limiting Reagent Questions today, and uncover new possibilities!
Frequently Asked Questions (FAQs)
-
Are the printables you get for free absolutely free?
- Yes they are! You can download and print these tools for free.
-
Can I download free printables for commercial uses?
- It's determined by the specific rules of usage. Be sure to read the rules of the creator before using their printables for commercial projects.
-
Do you have any copyright issues in Limiting Reagent Questions?
- Some printables may have restrictions on usage. Be sure to check the terms and regulations provided by the designer.
-
How do I print printables for free?
- You can print them at home using the printer, or go to a local print shop for high-quality prints.
-
What program is required to open Limiting Reagent Questions?
- Most printables come as PDF files, which is open with no cost software, such as Adobe Reader.
How To Calculate Limiting Reactant Slideshare
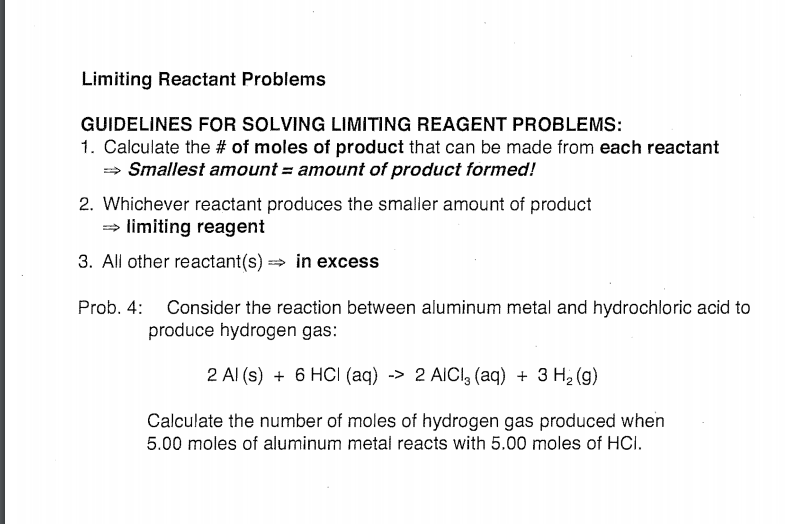
Visualizing Limiting Reactant 2 H 2 O 2
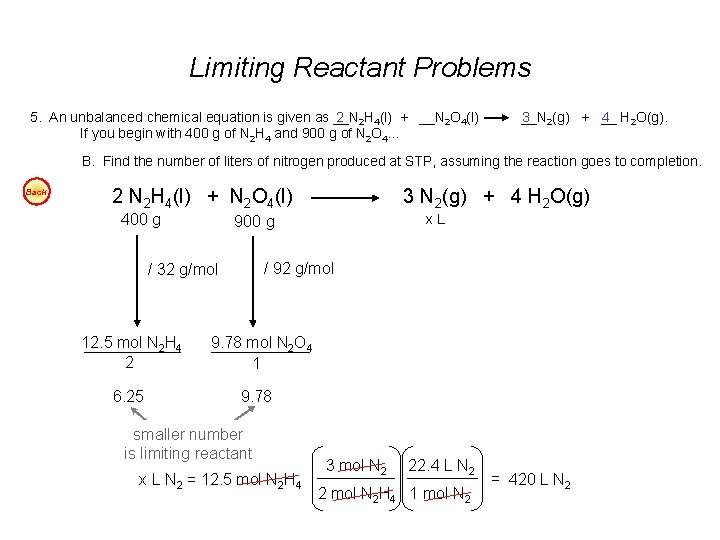
Check more sample of Limiting Reagent Questions below
Limiting Reagent Worksheet Doc Worksheet

How To Calculate The Limiting Reactant Calculate The Molecular Weight

How To Calculate Limiting Reactant Slideshare
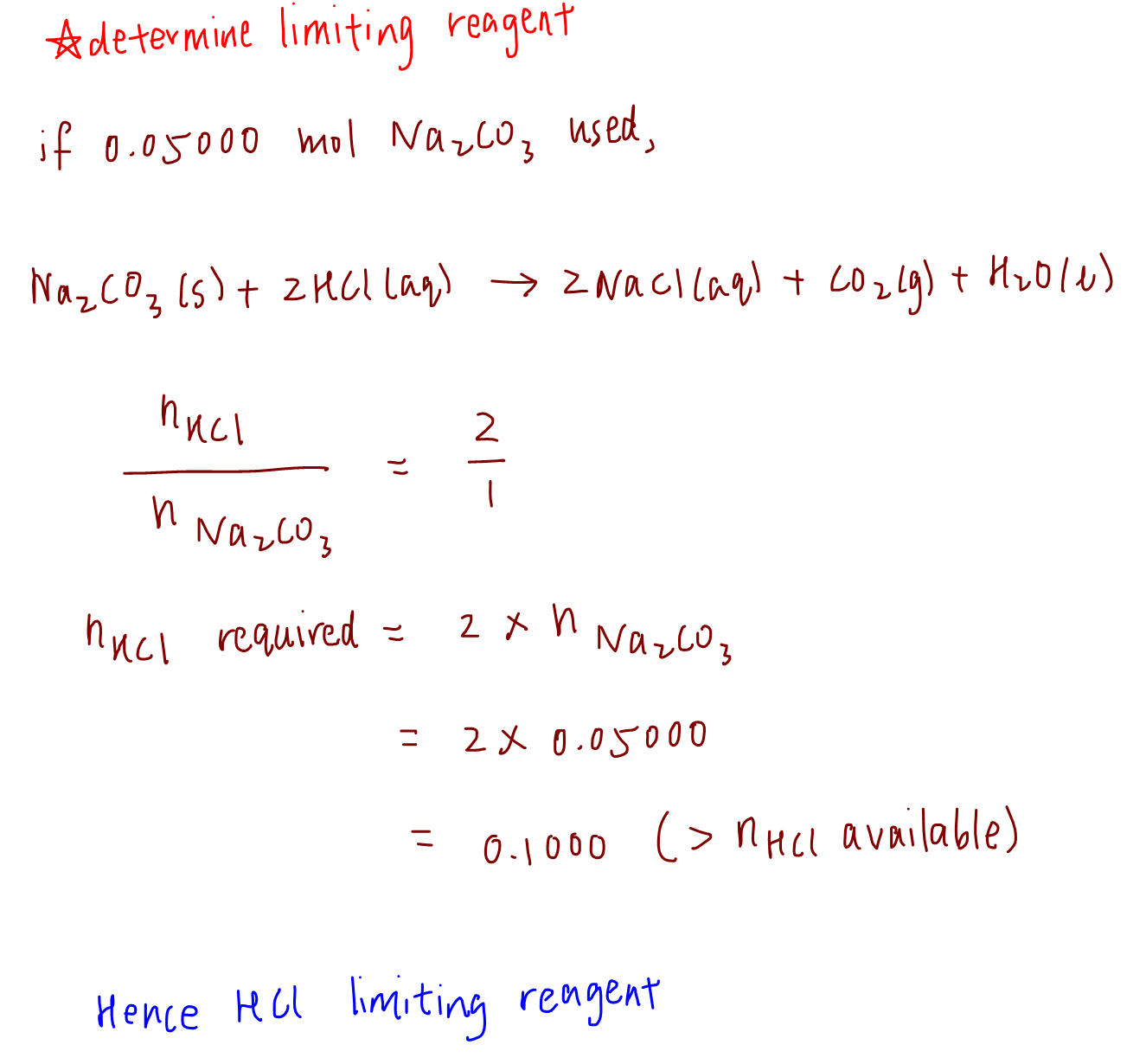
Stoichiometry Limiting Reagent Worksheet Answers

Class 11 Limiting Reagent Easiest Trick To Do Questions Of Limiting

Limiting Reagent Worksheet 2 Semesprit


https://www.chemteam.info/Stoichiometry/Limiting...
Which is the limiting reagent Solution path 1 1 Calculate moles of sucrose 10 0 g 342 2948 g mol 0 0292146 mol 2 Calculate moles of oxygen required to react with moles of sucrose From the coefficients we see that 12 moles of oxygen are require for every one mole of sucrose Therefore

https://mmerevise.co.uk/gcse-chemistry-revision/limiting-reactants
The reactant that is not in excess is known as the limiting reactant also known as the limiting reagent The limiting reactant is so called as it limits the amount of product that can be formed The amount of product formed will be directly proportional to the amount of limiting reactant used
Which is the limiting reagent Solution path 1 1 Calculate moles of sucrose 10 0 g 342 2948 g mol 0 0292146 mol 2 Calculate moles of oxygen required to react with moles of sucrose From the coefficients we see that 12 moles of oxygen are require for every one mole of sucrose Therefore
The reactant that is not in excess is known as the limiting reactant also known as the limiting reagent The limiting reactant is so called as it limits the amount of product that can be formed The amount of product formed will be directly proportional to the amount of limiting reactant used

Stoichiometry Limiting Reagent Worksheet Answers

How To Calculate The Limiting Reactant Calculate The Molecular Weight

Class 11 Limiting Reagent Easiest Trick To Do Questions Of Limiting

Limiting Reagent Worksheet 2 Semesprit

Limiting Reagent GCSE AQA Teaching Resources

Limiting Reagent Worksheets Answer Key

Limiting Reagent Worksheets Answer Key
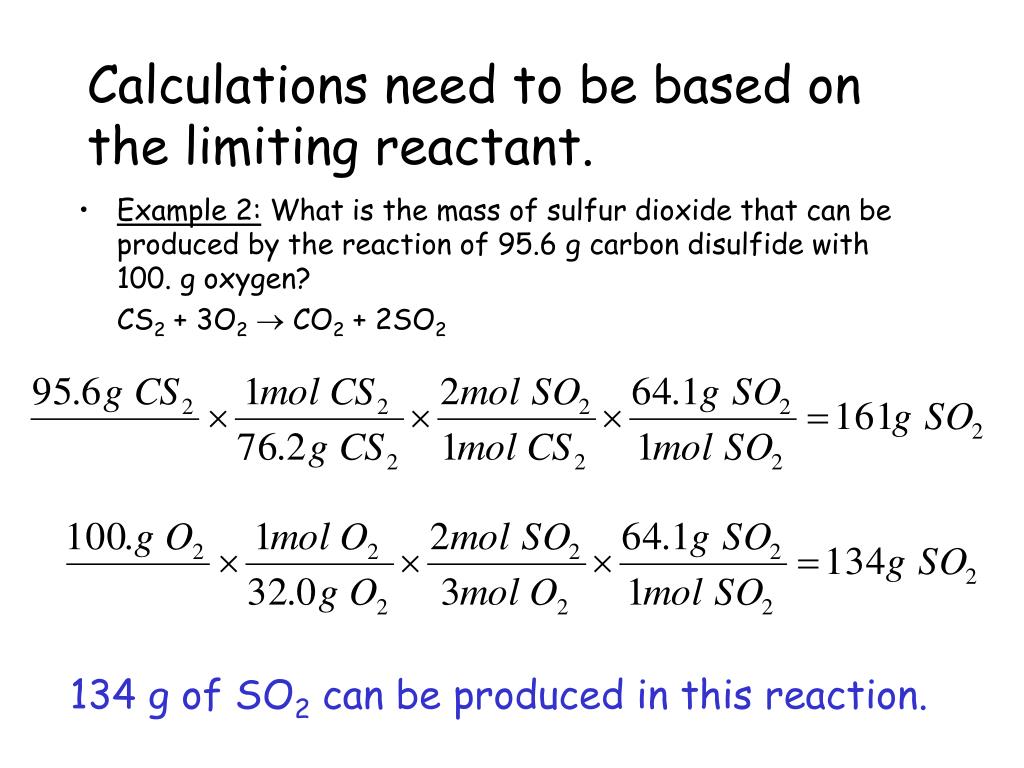
PPT Limiting Reactants Reagents PowerPoint Presentation Free