In the digital age, where screens have become the dominant feature of our lives but the value of tangible printed materials hasn't faded away. Whatever the reason, whether for education such as creative projects or simply adding an individual touch to the area, Lewis Structure Rules And Exceptions are now an essential resource. The following article is a dive into the world "Lewis Structure Rules And Exceptions," exploring what they are, how to find them, and the ways that they can benefit different aspects of your lives.
Get Latest Lewis Structure Rules And Exceptions Below

Lewis Structure Rules And Exceptions
Lewis Structure Rules And Exceptions -
Lecture notes on breakdown of the octet rule odd number of valence electrons octet deficient molecules valence shell expansion ionic bonds and polar covalent bonds and polar molecules
Write Lewis symbols for neutral atoms and ions Draw Lewis structures depicting the bonding in simple molecules Understand the proper use of the octet rule to predict bonding in simple molecules Thus far we have discussed the various types of bonds that form between atoms and or ions
Lewis Structure Rules And Exceptions include a broad selection of printable and downloadable material that is available online at no cost. They are available in numerous forms, like worksheets templates, coloring pages, and much more. The beauty of Lewis Structure Rules And Exceptions lies in their versatility and accessibility.
More of Lewis Structure Rules And Exceptions
Exceptions To The Octet Rule Lewis Dot Diagrams YouTube

Exceptions To The Octet Rule Lewis Dot Diagrams YouTube
Exceptions to the Octet Rule Many covalent molecules have central atoms that do not have eight electrons in their Lewis structures These molecules fall into three categories Odd electron molecules have an odd number of valence electrons and therefore have an unpaired electron
In all cases these bonds involve the sharing or transfer of valence shell electrons between atoms In this section we will explore the typical method for depicting valence shell electrons and chemical bonds namely Lewis symbols and Lewis structures
The Lewis Structure Rules And Exceptions have gained huge popularity due to a myriad of compelling factors:
-
Cost-Effective: They eliminate the necessity to purchase physical copies or costly software.
-
customization: There is the possibility of tailoring printed materials to meet your requirements, whether it's designing invitations, organizing your schedule, or decorating your home.
-
Educational Value Education-related printables at no charge offer a wide range of educational content for learners of all ages, which makes them an essential source for educators and parents.
-
Affordability: The instant accessibility to a plethora of designs and templates cuts down on time and efforts.
Where to Find more Lewis Structure Rules And Exceptions
Covalent Bonding And Lewis Structures Exceptions To The Octet

Covalent Bonding And Lewis Structures Exceptions To The Octet
Lewis dot structures provide a simple model for rationalizing the bonding in most known compounds However there are three general exceptions to the octet rule Molecules such as NO with an odd number of electrons
Learn Lewis Dot Structures Exceptions with free step by step video explanations and practice problems by experienced tutors
Now that we've ignited your curiosity about Lewis Structure Rules And Exceptions Let's take a look at where the hidden treasures:
1. Online Repositories
- Websites like Pinterest, Canva, and Etsy offer a vast selection of printables that are free for a variety of uses.
- Explore categories like the home, decor, the arts, and more.
2. Educational Platforms
- Forums and websites for education often offer worksheets with printables that are free for flashcards, lessons, and worksheets. tools.
- Great for parents, teachers and students looking for additional resources.
3. Creative Blogs
- Many bloggers post their original designs as well as templates for free.
- The blogs covered cover a wide spectrum of interests, from DIY projects to party planning.
Maximizing Lewis Structure Rules And Exceptions
Here are some innovative ways ensure you get the very most of printables that are free:
1. Home Decor
- Print and frame stunning art, quotes, or decorations for the holidays to beautify your living spaces.
2. Education
- Use these printable worksheets free of charge to reinforce learning at home as well as in the class.
3. Event Planning
- Invitations, banners as well as decorations for special occasions such as weddings, birthdays, and other special occasions.
4. Organization
- Keep your calendars organized by printing printable calendars for to-do list, lists of chores, and meal planners.
Conclusion
Lewis Structure Rules And Exceptions are a treasure trove of practical and innovative resources designed to meet a range of needs and interests. Their availability and versatility make them a valuable addition to each day life. Explore the vast array of Lewis Structure Rules And Exceptions to open up new possibilities!
Frequently Asked Questions (FAQs)
-
Are printables actually absolutely free?
- Yes, they are! You can download and print these items for free.
-
Can I utilize free printing templates for commercial purposes?
- It's determined by the specific terms of use. Always verify the guidelines of the creator before utilizing printables for commercial projects.
-
Do you have any copyright violations with printables that are free?
- Some printables may contain restrictions in their usage. Check the terms and conditions provided by the author.
-
How do I print printables for free?
- You can print them at home using the printer, or go to an in-store print shop to get higher quality prints.
-
What software is required to open Lewis Structure Rules And Exceptions?
- The majority of printed documents are in the format of PDF, which can be opened with free software, such as Adobe Reader.
Lewis Structures Exceptions To The Octet Rule YouTube

Exceptions To The Octet Rule And Formal Charge YouTube

Check more sample of Lewis Structure Rules And Exceptions below
Lewis Structure Rules 2nd Period YouTube

Exceptions To The Octet Rule Practice Problems Examples Summary YouTube

Exceptions To The Octet Rule AP Chemistry Khan Academy YouTube

CHEMISTRY 101 Lewis Structures Exceptions To The Octet Rule YouTube

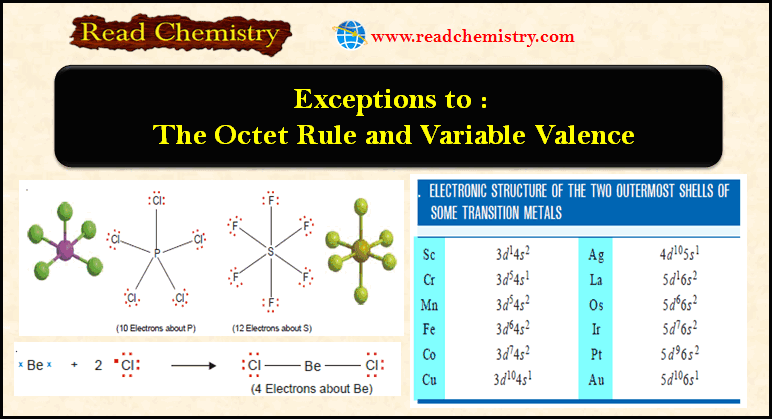
Exceptions To The Octet Rule And Variable Valence Read Chemistry
Evidence Hearsay Flow Chart Hot Sex Picture

https://chem.libretexts.org/Courses/Bellarmine...
Write Lewis symbols for neutral atoms and ions Draw Lewis structures depicting the bonding in simple molecules Understand the proper use of the octet rule to predict bonding in simple molecules Thus far we have discussed the various types of bonds that form between atoms and or ions

https://www.thoughtco.com/exceptions-to-the-octet-rule-603993
While Lewis electron dot structures help determine bonding in most compounds there are three general exceptions molecules in which atoms have fewer than eight electrons boron chloride and lighter s and p block elements molecules in which atoms have more than eight electrons sulfur hexafluoride and elements beyond period
Write Lewis symbols for neutral atoms and ions Draw Lewis structures depicting the bonding in simple molecules Understand the proper use of the octet rule to predict bonding in simple molecules Thus far we have discussed the various types of bonds that form between atoms and or ions
While Lewis electron dot structures help determine bonding in most compounds there are three general exceptions molecules in which atoms have fewer than eight electrons boron chloride and lighter s and p block elements molecules in which atoms have more than eight electrons sulfur hexafluoride and elements beyond period

CHEMISTRY 101 Lewis Structures Exceptions To The Octet Rule YouTube

Exceptions To The Octet Rule Practice Problems Examples Summary YouTube

Exceptions To The Octet Rule And Variable Valence Read Chemistry

Evidence Hearsay Flow Chart Hot Sex Picture

Which Elements Don T Follow The Octet Rule

How To Draw A Lewis Structure Chemistry Education Chemistry Lessons

How To Draw A Lewis Structure Chemistry Education Chemistry Lessons
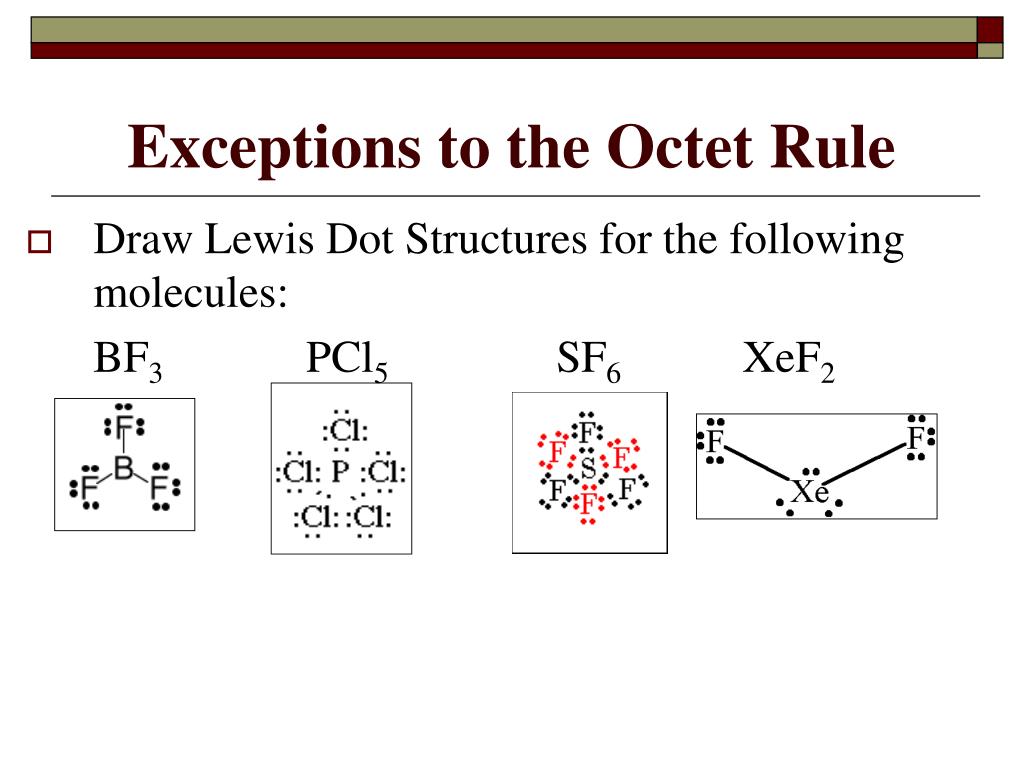
Rules Drawing Lewis Dot Structures