In this day and age where screens have become the dominant feature of our lives and our lives are dominated by screens, the appeal of tangible printed objects isn't diminished. For educational purposes project ideas, artistic or just adding an extra personal touch to your space, Lewis Dot Structure Of Lithium Chloride are now an essential resource. Through this post, we'll take a dive deep into the realm of "Lewis Dot Structure Of Lithium Chloride," exploring what they are, where they are available, and the ways that they can benefit different aspects of your daily life.
Get Latest Lewis Dot Structure Of Lithium Chloride Below

Lewis Dot Structure Of Lithium Chloride
Lewis Dot Structure Of Lithium Chloride -
Lewis structures are shown as element symbols with black dots shown around the symbol the number of black dots corresponding to the valence number Helium is H e with two black dots to the right Chlorine is C l with 2 on the left top and the right with 1 single dot at the bottom
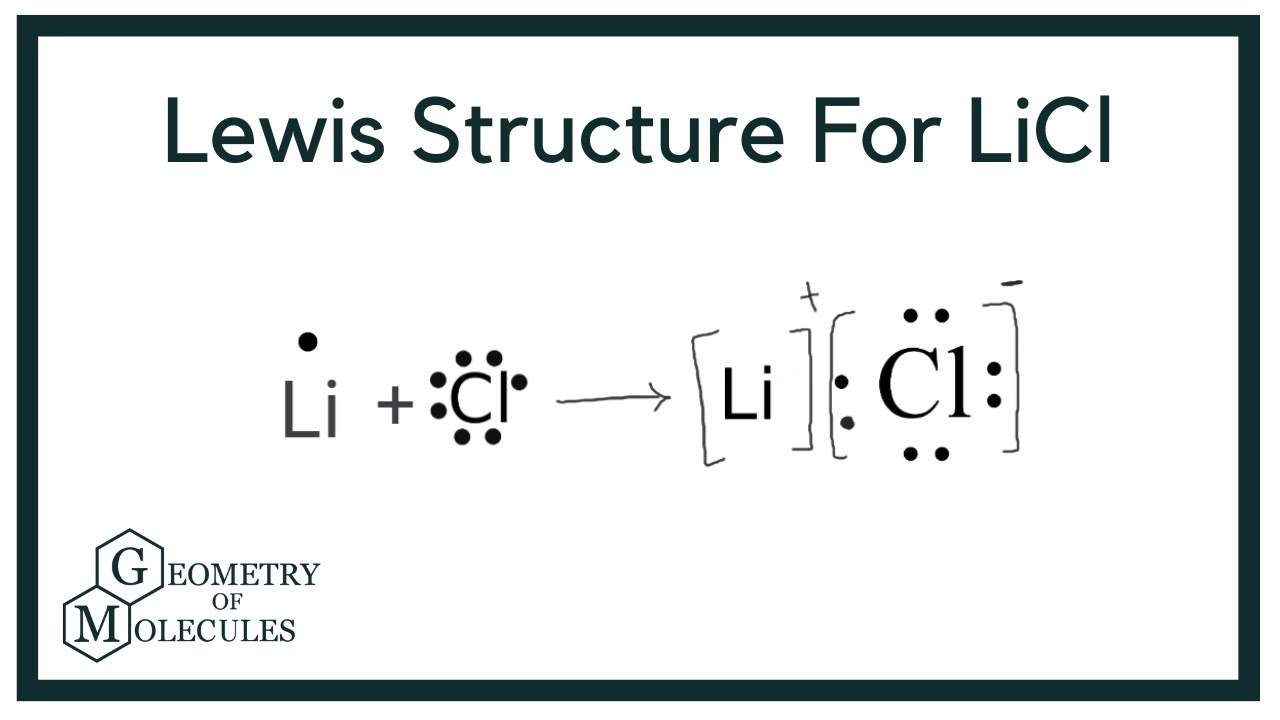
Lithium a metal in Group 1 loses one electron to become a 1 ion Chlorine a non metal in Group 17 gains one electron to become a 1 ion Together they combine to form a unit of LiCl lithium
Lewis Dot Structure Of Lithium Chloride cover a large collection of printable documents that can be downloaded online at no cost. They are available in numerous forms, including worksheets, coloring pages, templates and more. The appeal of printables for free is in their versatility and accessibility.
More of Lewis Dot Structure Of Lithium Chloride
This Question Is About Metal Compounds a Lithium Reacts With

This Question Is About Metal Compounds a Lithium Reacts With
A Lewis electron dot diagram or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element The number of dots equals the number of valence electrons in the atom
Lewis Structures We also use Lewis symbols to indicate the formation of covalent bonds which are shown in Lewis structures drawings that describe the bonding in molecules and polyatomic ions For example when two chlorine atoms form a chlorine molecule they share one pair of electrons
Printables for free have gained immense popularity for several compelling reasons:
-
Cost-Effective: They eliminate the need to buy physical copies or expensive software.
-
Customization: The Customization feature lets you tailor printables to fit your particular needs whether you're designing invitations or arranging your schedule or decorating your home.
-
Educational Worth: Printing educational materials for no cost provide for students from all ages, making them an essential tool for parents and teachers.
-
Accessibility: You have instant access a plethora of designs and templates helps save time and effort.
Where to Find more Lewis Dot Structure Of Lithium Chloride
Pin On Lewis Structure Chemistry

Pin On Lewis Structure Chemistry
Here s some of the guidelines for drawing dot structures So let s say we wanted to draw the dot structure for this molecule so silicon tetrafluoride The first thing we would need to do is to find the total number of valence electrons And we would account for these valence electrons in our dot structure
For example hydrogen and chlorine each need one more electron to achieve a noble gas configuration By sharing valence electrons each atom donates one the stable HCl HCl molecule is formed We will use a simplified representation of covalent bonds known as Lewis structures
Now that we've ignited your interest in printables for free we'll explore the places you can get these hidden treasures:
1. Online Repositories
- Websites like Pinterest, Canva, and Etsy provide an extensive selection of Lewis Dot Structure Of Lithium Chloride to suit a variety of objectives.
- Explore categories such as decoration for your home, education, crafting, and organization.
2. Educational Platforms
- Forums and educational websites often offer free worksheets and worksheets for printing along with flashcards, as well as other learning tools.
- Great for parents, teachers, and students seeking supplemental resources.
3. Creative Blogs
- Many bloggers share their innovative designs and templates for free.
- The blogs covered cover a wide variety of topics, everything from DIY projects to planning a party.
Maximizing Lewis Dot Structure Of Lithium Chloride
Here are some ways how you could make the most use of Lewis Dot Structure Of Lithium Chloride:
1. Home Decor
- Print and frame gorgeous artwork, quotes, or seasonal decorations that will adorn your living areas.
2. Education
- Use printable worksheets from the internet to enhance learning at home either in the schoolroom or at home.
3. Event Planning
- Designs invitations, banners and other decorations for special occasions like weddings or birthdays.
4. Organization
- Keep track of your schedule with printable calendars along with lists of tasks, and meal planners.
Conclusion
Lewis Dot Structure Of Lithium Chloride are an abundance with useful and creative ideas for a variety of needs and needs and. Their accessibility and versatility make they a beneficial addition to the professional and personal lives of both. Explore the vast array of Lewis Dot Structure Of Lithium Chloride to uncover new possibilities!
Frequently Asked Questions (FAQs)
-
Are Lewis Dot Structure Of Lithium Chloride truly for free?
- Yes they are! You can download and print these materials for free.
-
Can I use the free printing templates for commercial purposes?
- It's based on specific conditions of use. Make sure you read the guidelines for the creator before using their printables for commercial projects.
-
Are there any copyright violations with Lewis Dot Structure Of Lithium Chloride?
- Some printables may come with restrictions on use. You should read the terms and conditions offered by the designer.
-
How can I print printables for free?
- Print them at home with any printer or head to any local print store for better quality prints.
-
What software do I need in order to open Lewis Dot Structure Of Lithium Chloride?
- Most PDF-based printables are available in PDF format, which is open with no cost software such as Adobe Reader.
Draw The Lewis Structure Of Li2S lithium Sulfide YouTube

Lewis Structure For LiCl How To Draw The Lewis Structure For LiCl
Check more sample of Lewis Dot Structure Of Lithium Chloride below
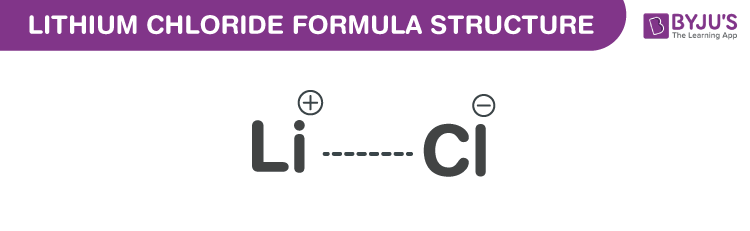
Lithium Chloride Formula Chemical Formula Structural Composition And
Atomic Structure Lewis Dot Diagram Lithium Stock Vector Royalty Free

Lewis Dot Diagram For Lithium Wiring Database My XXX Hot Girl
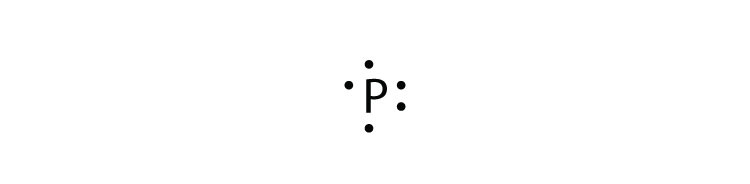
Lithium Chloride YouTube

Lewis Structure Magnesium Chloride Electron Diagram PNG 1024x1024px
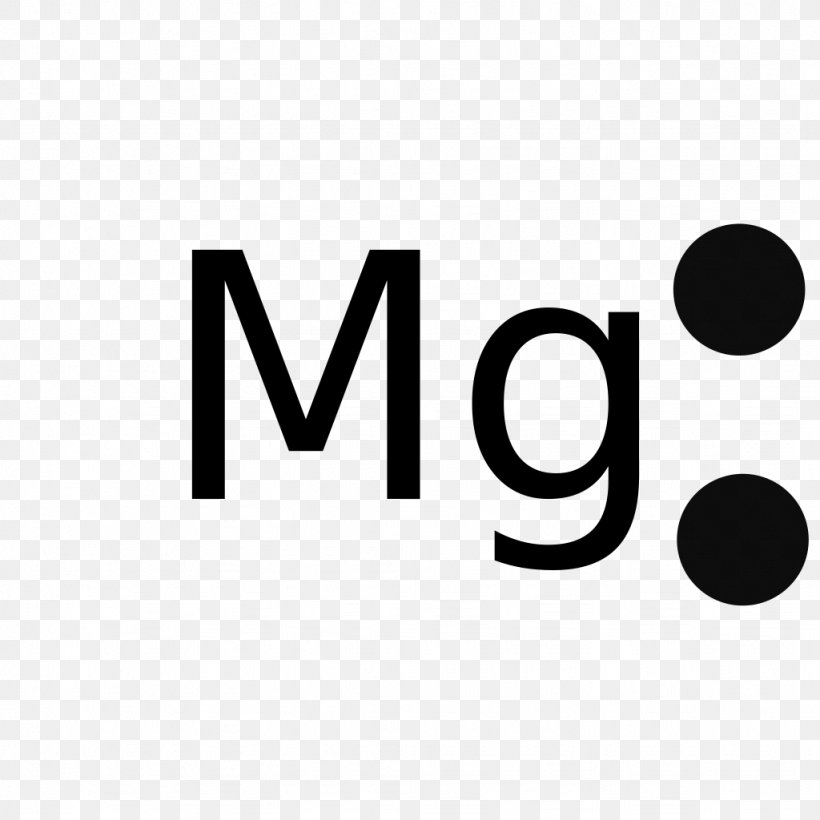
Draw The Lewis Structure Of Li2O lithium Oxide YouTube


https://www.youtube.com/watch?v=TghEJyg3P64
Lithium a metal in Group 1 loses one electron to become a 1 ion Chlorine a non metal in Group 17 gains one electron to become a 1 ion Together they combine to form a unit of LiCl lithium

https://www.wolframalpha.com/widgets/view.jsp?id=...
This widget gets the Lewis structure of chemical compounds Get the free Lewis Structure Finder widget for your website blog Wordpress Blogger or iGoogle Find more Chemistry widgets in Wolfram Alpha
Lithium a metal in Group 1 loses one electron to become a 1 ion Chlorine a non metal in Group 17 gains one electron to become a 1 ion Together they combine to form a unit of LiCl lithium
This widget gets the Lewis structure of chemical compounds Get the free Lewis Structure Finder widget for your website blog Wordpress Blogger or iGoogle Find more Chemistry widgets in Wolfram Alpha

Lithium Chloride YouTube

Atomic Structure Lewis Dot Diagram Lithium Stock Vector Royalty Free
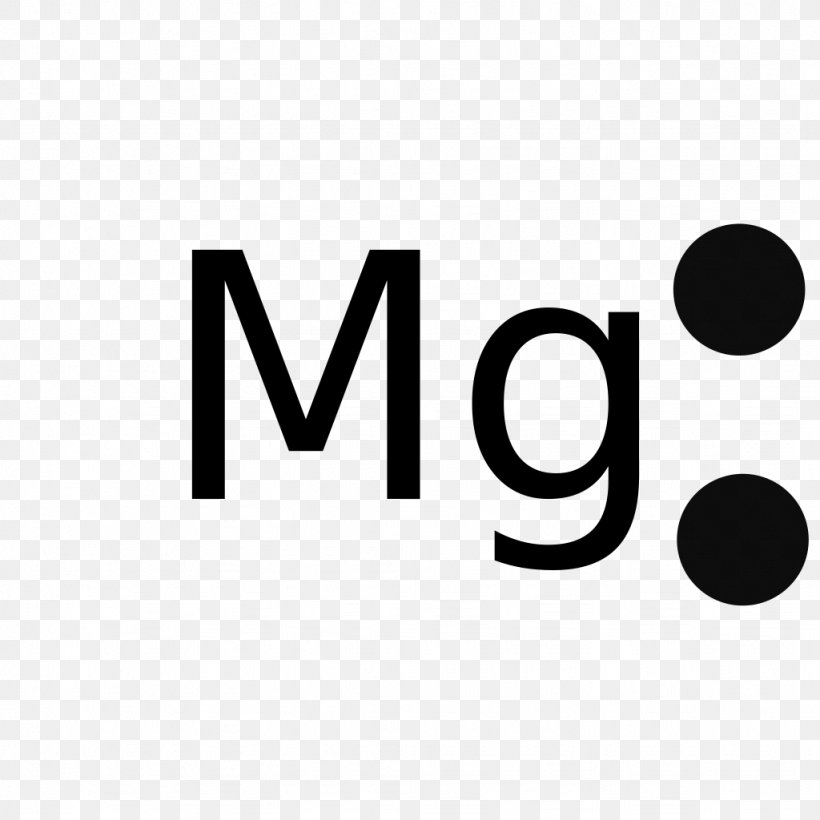
Lewis Structure Magnesium Chloride Electron Diagram PNG 1024x1024px

Draw The Lewis Structure Of Li2O lithium Oxide YouTube

Ionic Bonding GCSE Chemistry Combined Science AQA Revision Study
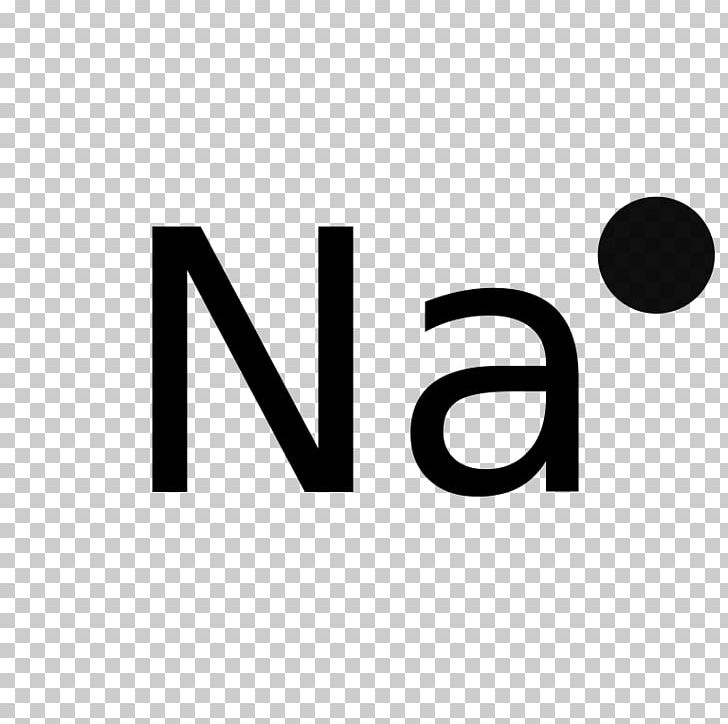
Lewis Structure Sodium Diagram Electron Chloride PNG Clipart Angle
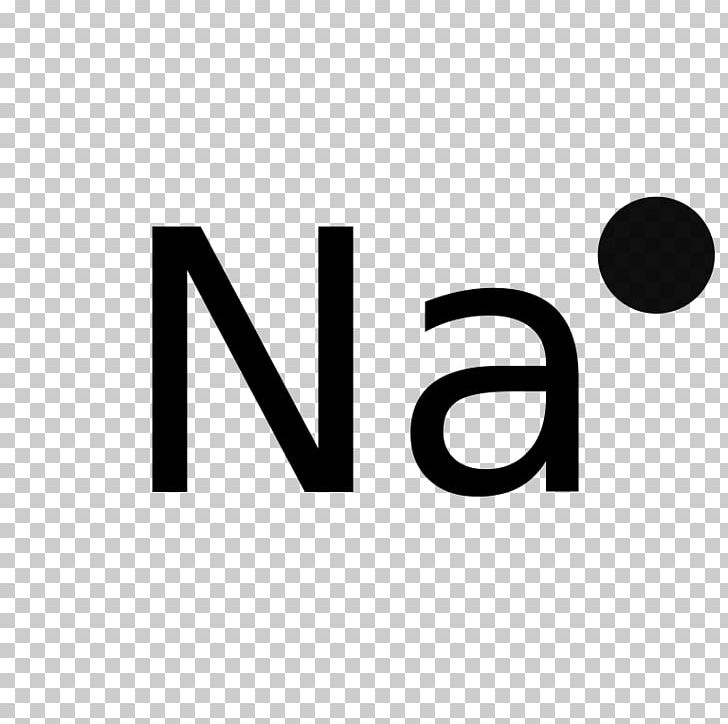
Lewis Structure Sodium Diagram Electron Chloride PNG Clipart Angle

Help Me With Basic Chemistry How To Do Lewis Dot Structure Simple