In the age of digital, with screens dominating our lives and the appeal of physical printed items hasn't gone away. No matter whether it's for educational uses and creative work, or simply to add a personal touch to your space, How Does Polarity Affect Solubility are a great source. In this article, we'll take a dive through the vast world of "How Does Polarity Affect Solubility," exploring the benefits of them, where to find them, and how they can add value to various aspects of your daily life.
Get Latest How Does Polarity Affect Solubility Below

How Does Polarity Affect Solubility
How Does Polarity Affect Solubility -
In general polar substances will dissolve other polar substances while nonpolar materials will dissolve other nonpolar materials The greater the difference in molecular structure and hence in intermolecular attractions the lower the mutual solubility
Because water is polar substances that are polar or ionic will dissolve in it Because of the shape of the molecule and the polar OH grouping in methanol we expect its molecules to be polar and for it to be soluble in water
Printables for free cover a broad range of printable, free materials that are accessible online for free cost. They are available in a variety of types, such as worksheets coloring pages, templates and much more. The attraction of printables that are free lies in their versatility and accessibility.
More of How Does Polarity Affect Solubility
PPT Ch 10 PowerPoint Presentation Free Download ID 3538935

PPT Ch 10 PowerPoint Presentation Free Download ID 3538935
Substances with similar polarities tend to be soluble in one another like dissolves like Nonpolar substances are generally more soluble in nonpolar solvents while polar and ionic substances are generally more soluble in polar solvents
If the solvent is polar like water then a larger dipole moment indicating greater molecular polarity will tend to increase the solubility of a substance in it If the solvent is non polar like the hydrocarbon hexane then the exact opposite is true
How Does Polarity Affect Solubility have gained immense popularity due to a variety of compelling reasons:
-
Cost-Efficiency: They eliminate the necessity to purchase physical copies or expensive software.
-
The ability to customize: They can make printed materials to meet your requirements be it designing invitations for your guests, organizing your schedule or even decorating your house.
-
Educational value: Educational printables that can be downloaded for free offer a wide range of educational content for learners of all ages. This makes them a great device for teachers and parents.
-
It's easy: Fast access numerous designs and templates, which saves time as well as effort.
Where to Find more How Does Polarity Affect Solubility
PPT Chapter 10 Chemical Bonding II Molecular Shapes And Valence Bond Theory PowerPoint

PPT Chapter 10 Chemical Bonding II Molecular Shapes And Valence Bond Theory PowerPoint
About Transcript Organic compounds tend to dissolve well in solvents that have similar properties to themselves This principle is often referred to as like dissolves like which means that polar molecules will generally dissolve well in polar solvents and non polar molecules will generally dissolve in non polar solvents Questions
How does solubility change with polarity How does solubility affect chromatography How can solubility be changed How is solubility expressed in units of measurement
Now that we've piqued your interest in printables for free Let's see where you can discover these hidden treasures:
1. Online Repositories
- Websites such as Pinterest, Canva, and Etsy provide an extensive selection in How Does Polarity Affect Solubility for different objectives.
- Explore categories like home decor, education, organizing, and crafts.
2. Educational Platforms
- Educational websites and forums usually provide free printable worksheets Flashcards, worksheets, and other educational tools.
- Ideal for parents, teachers, and students seeking supplemental resources.
3. Creative Blogs
- Many bloggers share their imaginative designs and templates free of charge.
- These blogs cover a wide range of interests, including DIY projects to planning a party.
Maximizing How Does Polarity Affect Solubility
Here are some unique ways how you could make the most of How Does Polarity Affect Solubility:
1. Home Decor
- Print and frame stunning artwork, quotes or seasonal decorations to adorn your living areas.
2. Education
- Use these printable worksheets free of charge to build your knowledge at home, or even in the classroom.
3. Event Planning
- Design invitations, banners as well as decorations for special occasions like weddings or birthdays.
4. Organization
- Get organized with printable calendars or to-do lists. meal planners.
Conclusion
How Does Polarity Affect Solubility are a treasure trove with useful and creative ideas that satisfy a wide range of requirements and needs and. Their access and versatility makes them an invaluable addition to every aspect of your life, both professional and personal. Explore the plethora of How Does Polarity Affect Solubility and uncover new possibilities!
Frequently Asked Questions (FAQs)
-
Are printables available for download really gratis?
- Yes you can! You can print and download the resources for free.
-
Can I use the free printables for commercial uses?
- It's based on specific usage guidelines. Always verify the guidelines of the creator before utilizing printables for commercial projects.
-
Do you have any copyright issues in printables that are free?
- Certain printables may be subject to restrictions in their usage. Be sure to read the terms and condition of use as provided by the creator.
-
How can I print How Does Polarity Affect Solubility?
- Print them at home using an printer, or go to the local print shop for top quality prints.
-
What software do I need in order to open printables free of charge?
- Most PDF-based printables are available as PDF files, which is open with no cost programs like Adobe Reader.
How Does Polarity Affect Solubility Of Solutes In Liquid Solvents JacAnswers

Polarity And Solubility YouTube

Check more sample of How Does Polarity Affect Solubility below
PPT Chapter 12 Intermolecular Attractions And The Properties Of Liquids And Solids PowerPoint

Podcastppt5

How Does Molecular Shape Affect Polarity Explained With Examples
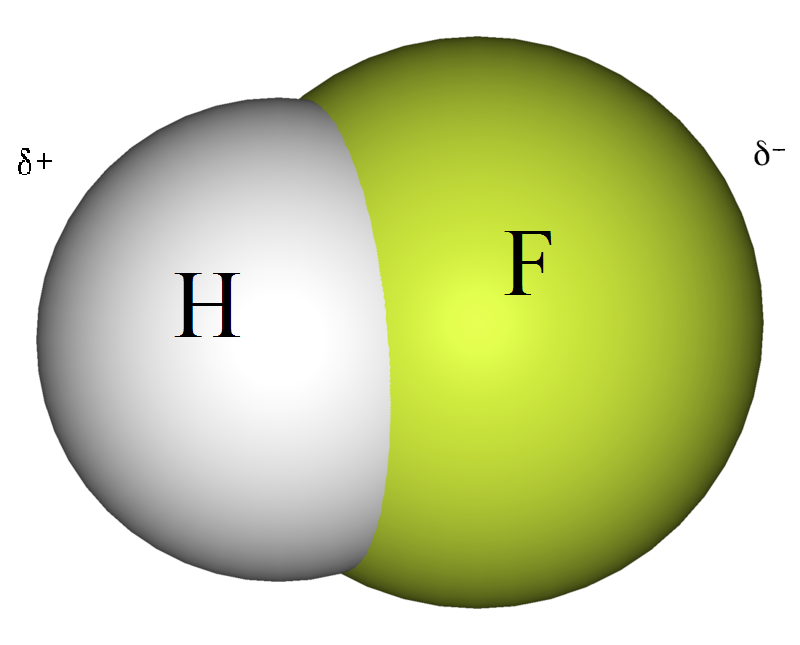
How Does The Polarity Of The Eluent And Sample Affect The Rf Value In Thin Layer Chromatography

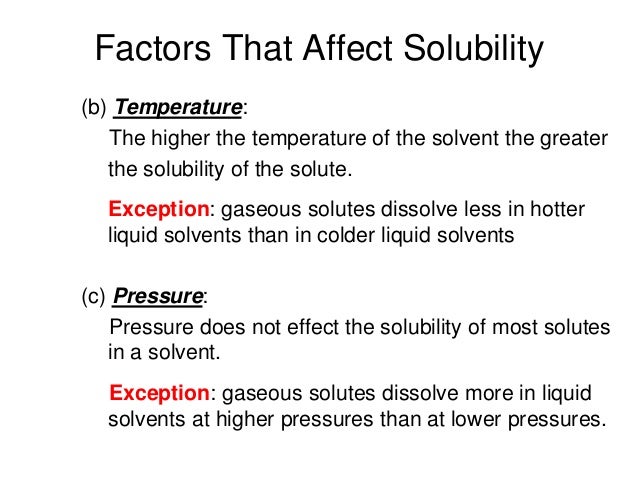
Factors That Affect Solubility
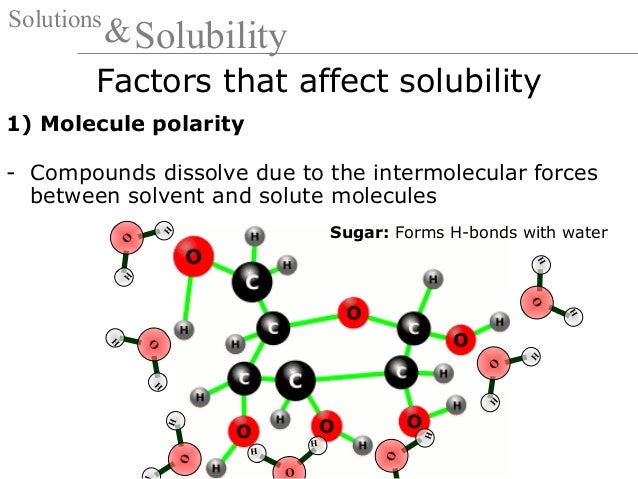
19 Solutions And Solubility

https://chem.libretexts.org/Courses/Anoka-Ramsey...
Because water is polar substances that are polar or ionic will dissolve in it Because of the shape of the molecule and the polar OH grouping in methanol we expect its molecules to be polar and for it to be soluble in water

https://socratic.org/questions/how-is-molecular-polarity-related-to-solubility
Thus polarity affects solubility If solute and solvent have approximately the same polarity they will probably form a solution Like dissolves like Polar solutes dissolve in polar solvents nonpolar solutes dissolve in nonpolar solvents
Because water is polar substances that are polar or ionic will dissolve in it Because of the shape of the molecule and the polar OH grouping in methanol we expect its molecules to be polar and for it to be soluble in water
Thus polarity affects solubility If solute and solvent have approximately the same polarity they will probably form a solution Like dissolves like Polar solutes dissolve in polar solvents nonpolar solutes dissolve in nonpolar solvents

How Does The Polarity Of The Eluent And Sample Affect The Rf Value In Thin Layer Chromatography

Podcastppt5

Factors That Affect Solubility

19 Solutions And Solubility

Question 7ba27 Example

How Does Temperature Affect Solubility YouTube

How Does Temperature Affect Solubility YouTube

Solved How Does The Pressure Affect The Solubility Of NH3 Chegg