In this age of electronic devices, with screens dominating our lives yet the appeal of tangible printed objects hasn't waned. In the case of educational materials and creative work, or simply to add the personal touch to your space, Difference Between Static And Dynamic Equilibrium In Chemistry are now an essential source. For this piece, we'll take a dive into the sphere of "Difference Between Static And Dynamic Equilibrium In Chemistry," exploring what they are, how to find them and how they can be used to enhance different aspects of your lives.
Get Latest Difference Between Static And Dynamic Equilibrium In Chemistry Below

Difference Between Static And Dynamic Equilibrium In Chemistry
Difference Between Static And Dynamic Equilibrium In Chemistry -
Static vs Dynamic Equilibrium What is a Static Equilibrium A static equilibrium is a state reached when a reaction goes to completion Figure 1 At this stage the rates of forward and reverse reaction both equal zero Figure 1 diagram illustrates an example of static equilibrium All reactants blue are converted into products yellow
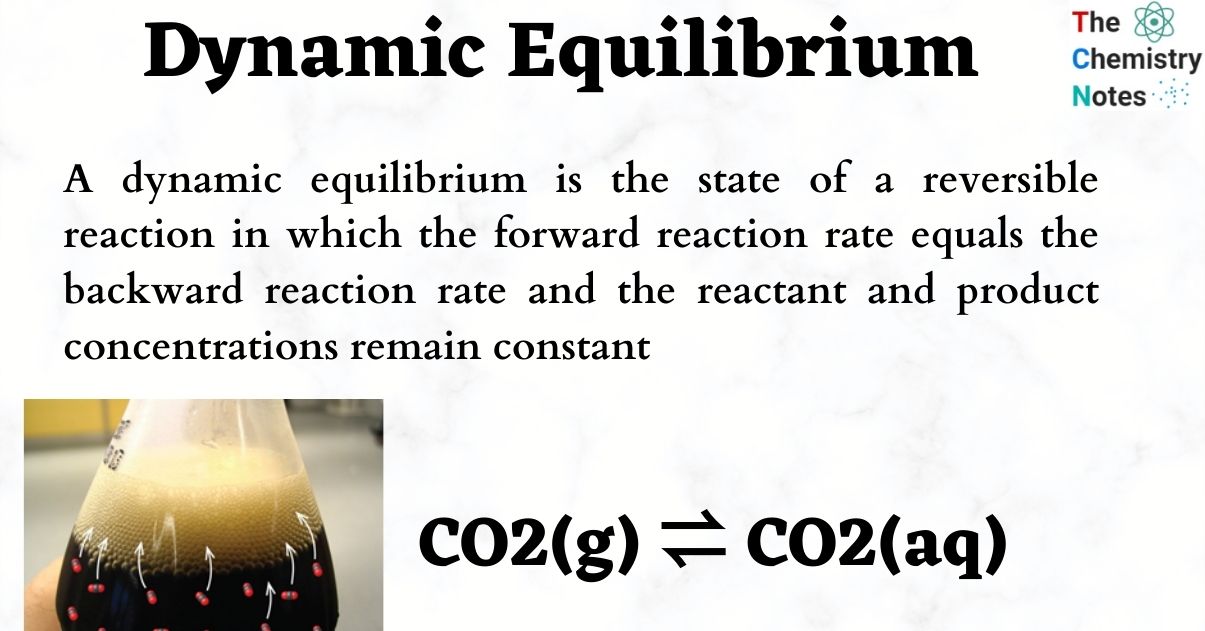
Dynamic equilibrium is the steady state of a reversible reaction where the rate of the forward reaction is the same as the reaction rate in the backward direction Static equilibrium also known as mechanical equilibrium means the reaction has stopped In other words the system is at rest
Printables for free cover a broad range of printable, free material that is available online at no cost. The resources are offered in a variety formats, such as worksheets, templates, coloring pages, and many more. The appeal of printables for free is their versatility and accessibility.
More of Difference Between Static And Dynamic Equilibrium In Chemistry
Chemical Equilibrium ClassNotes ng
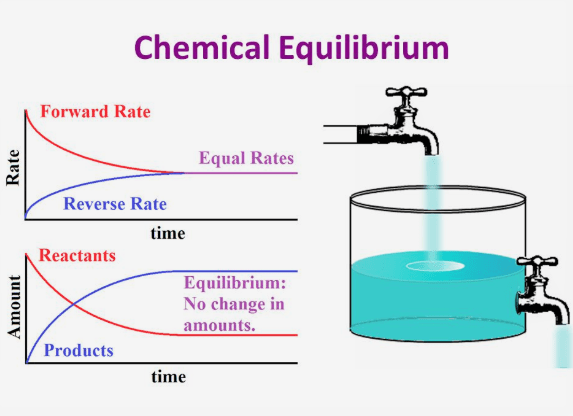
Chemical Equilibrium ClassNotes ng
Dynamic equilibrium explained with examples and a graph What does dynamic equilibrium mean Learn the difference between static and dynamic equilibrium
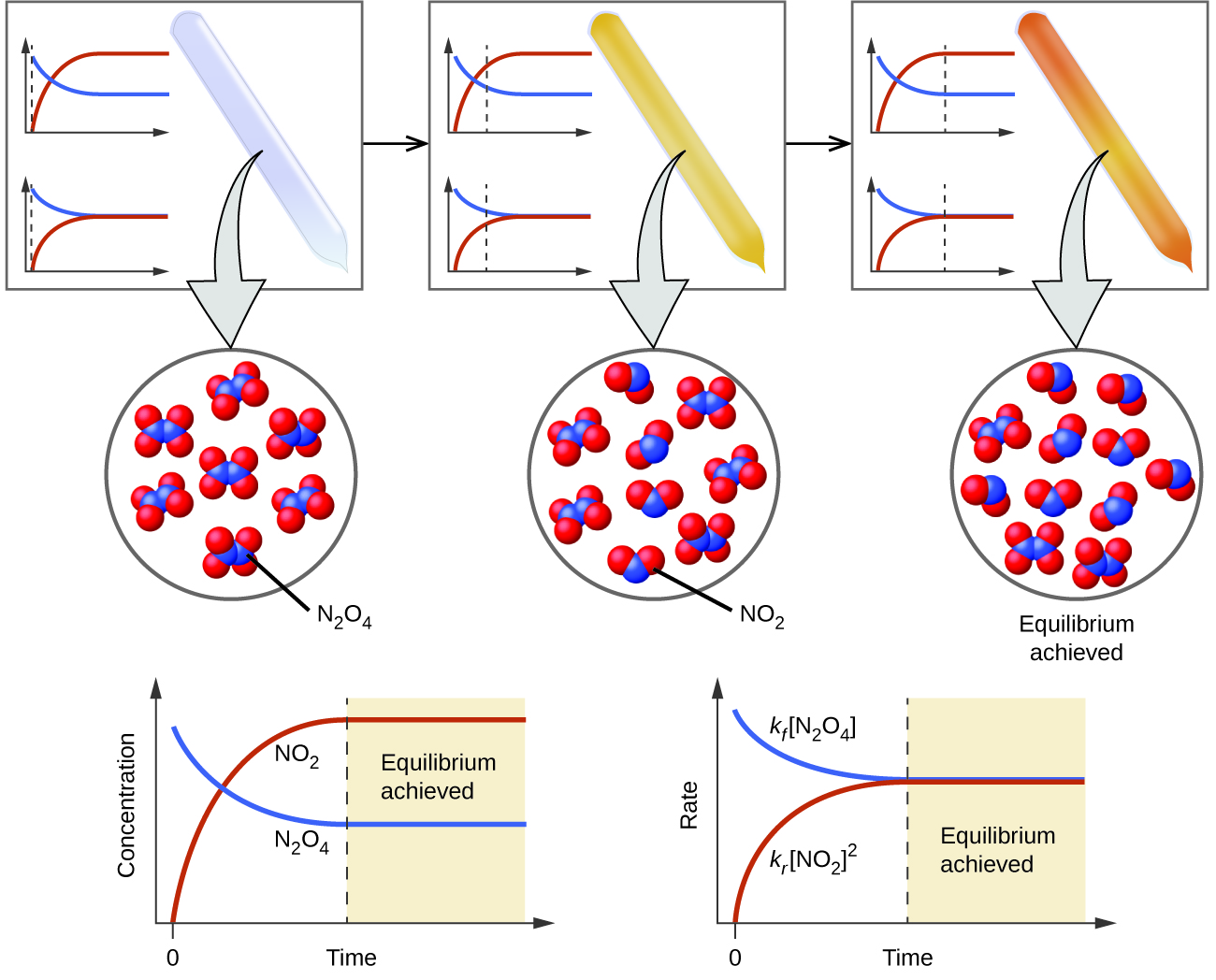
Equilibrium is macroscopically static but is microscopically dynamic To further illustrate the dynamic character of chemical equilibrium suppose that we now change the composition of the system previously at equilibrium by adding some C or withdrawing some A thus changing their active masses
Printables for free have gained immense popularity due to several compelling reasons:
-
Cost-Effective: They eliminate the need to purchase physical copies or costly software.
-
Personalization The Customization feature lets you tailor the design to meet your needs when it comes to designing invitations as well as organizing your calendar, or even decorating your home.
-
Educational Value Education-related printables at no charge cater to learners of all ages. This makes the perfect tool for parents and teachers.
-
Easy to use: Instant access to various designs and templates reduces time and effort.
Where to Find more Difference Between Static And Dynamic Equilibrium In Chemistry
Chemistry A Level Revision Equilibrium
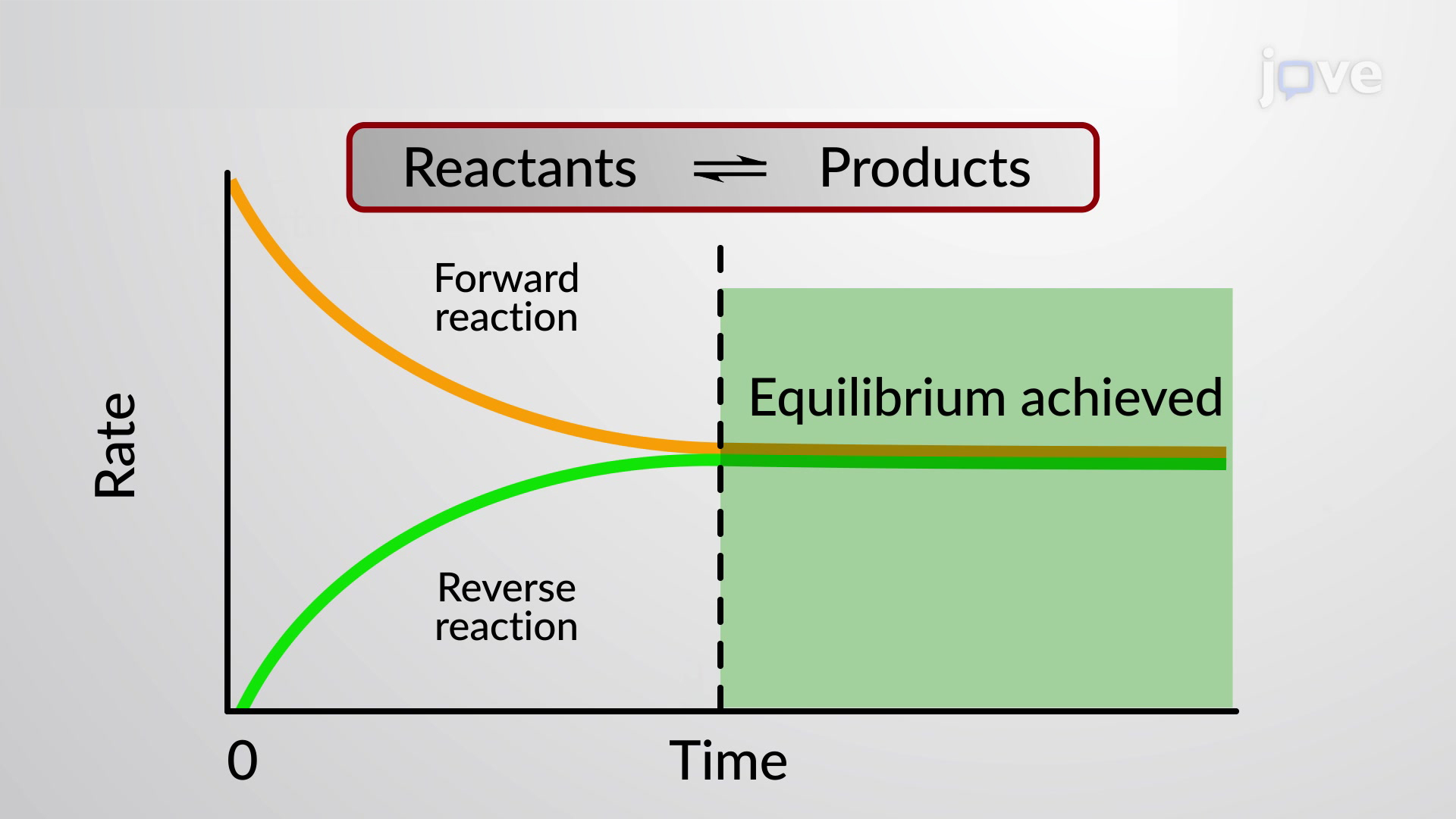
Chemistry A Level Revision Equilibrium
Flexi Says Static equilibrium refers to a system where there is no movement or change in the state of the system All forces and torques acting on the system are balanced and the system remains in a constant state In contrast dynamic equilibrium refers to a system where there is continuous movement or change but the overall state of the
Chemical equilibrium is a dynamic process that consists of a forward reaction in which reactants are converted to products and a reverse reaction in which products are converted to reactants At equilibrium the forward and reverse reactions proceed at equal rates
Now that we've piqued your curiosity about Difference Between Static And Dynamic Equilibrium In Chemistry Let's look into where the hidden gems:
1. Online Repositories
- Websites such as Pinterest, Canva, and Etsy offer a huge selection of Difference Between Static And Dynamic Equilibrium In Chemistry to suit a variety of reasons.
- Explore categories like interior decor, education, craft, and organization.
2. Educational Platforms
- Educational websites and forums frequently provide free printable worksheets including flashcards, learning tools.
- Ideal for parents, teachers and students in need of additional sources.
3. Creative Blogs
- Many bloggers share their imaginative designs and templates for free.
- These blogs cover a broad array of topics, ranging everything from DIY projects to planning a party.
Maximizing Difference Between Static And Dynamic Equilibrium In Chemistry
Here are some creative ways in order to maximize the use of printables for free:
1. Home Decor
- Print and frame gorgeous art, quotes, or seasonal decorations that will adorn your living areas.
2. Education
- Use these printable worksheets free of charge for reinforcement of learning at home, or even in the classroom.
3. Event Planning
- Designs invitations, banners as well as decorations for special occasions such as weddings, birthdays, and other special occasions.
4. Organization
- Get organized with printable calendars or to-do lists. meal planners.
Conclusion
Difference Between Static And Dynamic Equilibrium In Chemistry are an abundance of useful and creative resources catering to different needs and desires. Their accessibility and flexibility make these printables a useful addition to the professional and personal lives of both. Explore the vast collection of printables for free today and unlock new possibilities!
Frequently Asked Questions (FAQs)
-
Are the printables you get for free cost-free?
- Yes, they are! You can print and download the resources for free.
-
Can I use the free printouts for commercial usage?
- It is contingent on the specific conditions of use. Make sure you read the guidelines for the creator before utilizing their templates for commercial projects.
-
Are there any copyright concerns when using Difference Between Static And Dynamic Equilibrium In Chemistry?
- Certain printables could be restricted in use. You should read these terms and conditions as set out by the creator.
-
How can I print printables for free?
- You can print them at home using printing equipment or visit a local print shop to purchase higher quality prints.
-
What program will I need to access printables for free?
- Many printables are offered in the format of PDF, which is open with no cost programs like Adobe Reader.
What Is Meant By Static Balance And Dynamic Balance
13 1 Chemical Equilibria Chemistry 112 Chapters 12 17 Of OpenStax
Check more sample of Difference Between Static And Dynamic Equilibrium In Chemistry below
Static And Dynamic Equilibrium Explained With Their Differences

Static And Dynamic Equilibrium HubPages
7 Important Difference Between Static And Dynamic Equilibrium With

Wht Is Static And Dynamic Equilibrium Brainly in

Chemical Equilibrium I Types Of Equilibrium Equilibrium Constant

Dynamic Equilibrium Definition Important Examples

https://biologydictionary.net/difference-static-dynamic-equilibrium
Dynamic equilibrium is the steady state of a reversible reaction where the rate of the forward reaction is the same as the reaction rate in the backward direction Static equilibrium also known as mechanical equilibrium means the reaction has stopped In other words the system is at rest

https://thisvsthat.io/dynamic-equilibrium-vs-static
While both dynamic equilibrium and static equilibrium involve a state of balance there are several key differences between the two Dynamic equilibrium involves continuous change and movement while static equilibrium is characterized by stability and motionlessness
Dynamic equilibrium is the steady state of a reversible reaction where the rate of the forward reaction is the same as the reaction rate in the backward direction Static equilibrium also known as mechanical equilibrium means the reaction has stopped In other words the system is at rest
While both dynamic equilibrium and static equilibrium involve a state of balance there are several key differences between the two Dynamic equilibrium involves continuous change and movement while static equilibrium is characterized by stability and motionlessness

Wht Is Static And Dynamic Equilibrium Brainly in

Static And Dynamic Equilibrium HubPages

Chemical Equilibrium I Types Of Equilibrium Equilibrium Constant

Dynamic Equilibrium Definition Important Examples

Why Is Dynamic Used To Describe Chemical Equilibrium

Difference Between Chemical Equilibrium And Dynamic Equilibrium

Difference Between Chemical Equilibrium And Dynamic Equilibrium
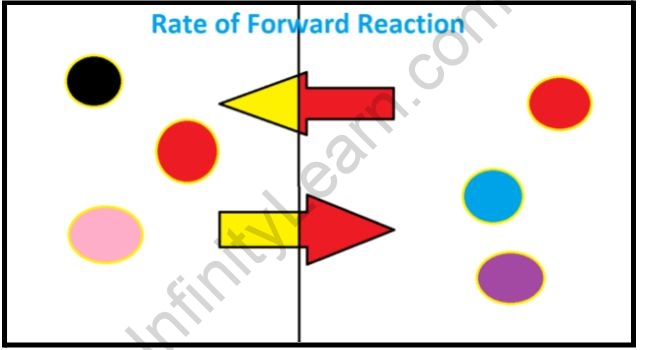
Dynamic Nature Of Equilibrium Infinity Learn By Sri Chaitanya